New perspectives for treating psychiatric disorders
Scientists have determined the structure of Glycine Transporter 1. The finding could open new avenues for developing therapeutics for psychiatric disorders
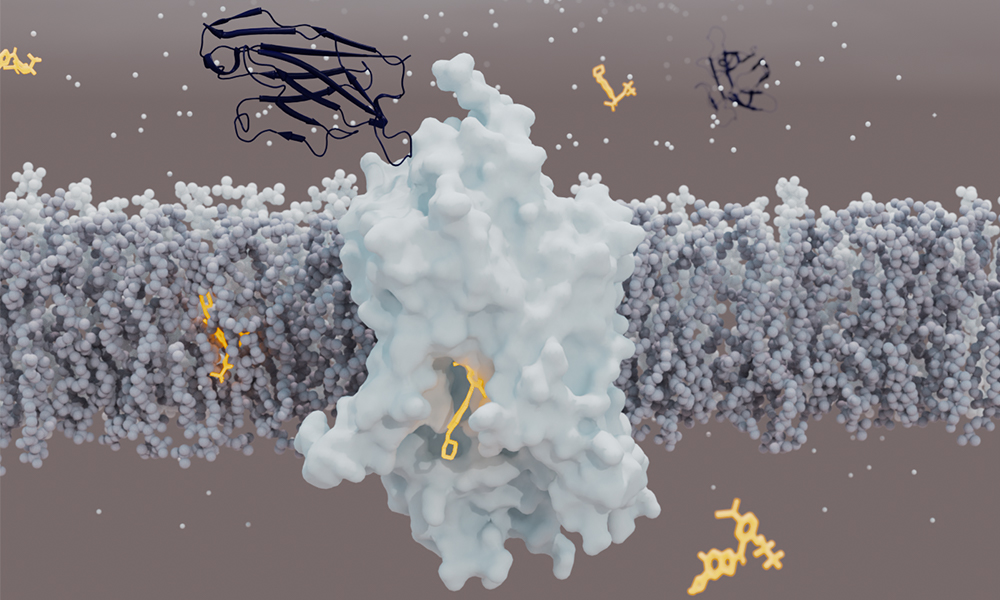
Glycine regulates neuronal activity in the brain
Glycine is the smallest amino acid – one of the building blocks of proteins. It acts also as a neurotransmitter in the brain, enabling neurons to communicate with each other and modulating neuronal activity. Many researchers have focused on increasing glycine levels in synapses to find an effective treatment for schizophrenia. This could be done using inhibitors targeting Glycine Transporter 1 (GlyT1), a protein that sits in neuronal cell membranes and is responsible for the uptake of glycine into neurons. However, the development of such drugs has been hampered because the 3D structure of GlyT1 was not known.
To determine the structure of GlyT1, researchers at the Danish Research Institute of Translational Neuroscience (DANDRITE), which is part of the Nordic EMBL Partnership for Molecular Medicine, F. Hoffmann-La Roche, EMBL Hamburg, the University of Zurich, Aarhus University, and Linkster Therapeutics joined forces. “This project required multidisciplinary collaboration and unique expertise from different labs over several years,” says Azadeh Shahsavar, first author of the study and now an assistant professor at DANDRITE. She performed the measurements for the study during her time as a postdoc in the EMBL Interdisciplinary Postdocs (EIPOD) programme, during which she worked at EMBL Hamburg, DANDRITE and Roche.
Poul Nissen, Director of DANDRITE and a senior researcher in the study, comments: “We are immensely grateful for the EMBL’s EIPOD scheme and the Nordic EMBL Partnership to keep us on track for so long and allow us to explore very difficult approaches. We would not have succeeded without it, and without Azadeh’s persistence of course!”
Overcoming challenges in studying Glycine Transporter 1
GlyT1 turned out to be particularly challenging to study, because it is unstable when extracted from the cell membrane. To stabilise it, scientists combined several approaches, such as creating more stable variants of the protein. To catch the transporter in a clinically relevant state, the team used a chemical created by Roche that binds and stabilises GlyT1 from the inside, and designed a synthetic mini-antibody (sybody) that binds it from the outside.
The scientists tested 960 different conditions and managed to obtain GlyT1 crystals in one of them. “The crystals were very small and difficult to image. We chose to measure them at EMBL Hamburg’s beamline P14, which is well suited for challenging experiments like this one,” says Azadeh. The X-ray beam at P14 is particularly strong and focused, and its equipment has features tailored for work with even micrometre-sized crystals. Yet the quality of the crystals was variable, which made data collection challenging. Eventually, Azadeh’s perseverance paid off. “I remember when I saw electron density of the inhibitor for the first time. I was so excited, I couldn’t sleep for two nights,” she says. “You live for those rewarding moments.”
The final challenge was the data analysis. While the crystals were giving only weak diffraction patterns due to their small size, the strong X-rays destroyed the crystals in less than a second. A single crystal would yield only partial information about the structure, so Azadeh had to collect data from hundreds of crystals. “Processing such a huge amount of data was possible thanks to the unique infrastructure at EMBL Hamburg,” she says. Combining partial datasets was complex for the existing software, but the Schneider group at EMBL Hamburg wrote software specifically designed for such cases. It enabled Azadeh to merge datasets into a full picture of GlyT1 at 3.4 Å resolution (1 Å, or ångström, is one ten-billionth of a metre – about the size of a typical atom). “I really enjoyed working with people with different scientific backgrounds. Everybody contributed their unique expertise that made this study possible,” says Azadeh.
For Thomas Schneider, Joint Head of Research Infrastructures at EMBL Hamburg, the study is a perfect example highlighting the importance of both scientific excellence and the availability of cutting-edge infrastructures for progressing research. “For challenging projects like this, we are happy to put the methodological expertise of our staff to work and to make full use of the technological capabilities of our beamlines and sample preparation facilities. The high-intensity microfocused beam produced by the PETRA III synchrotron on the DESY campus and the versatile high-precision diffractometer that was developed in a collaboration between EMBL Hamburg, EMBL Grenoble, and ARINAX were key for this project.”
Azadeh agrees. “The excellence, infrastructure, hardware, and software provided by EMBL are of the highest quality, and they are constantly being improved,” she adds.
Blueprint for new therapeutics
The analysis revealed an unexpected structure of GlyT1. In contrast to other neurotransmitter transporters, which are bound by their inhibitors from the outer side of the cell membrane, GlyT1 is bound by its inhibitor from the inner side. “The structure was a surprise for us. It seems that the GlyT1 inhibitor must first cross the cell membranes before it can access GlyT1 from the inside of the neurons,” says Roger Dawson, a senior author in the study.
“This structure provides a blueprint for developing new inhibitors of GlyT1, be they organic molecules or antibodies,” explains Roger. “The sybody developed for this study binds GlyT1 at a previously unknown binding site and locks it in a state in which it cannot transport glycine any more. We could use this knowledge to develop drugs targeting not only GlyT1, but also other membrane transport proteins in the future.”
Azadeh Shahsavar was supported by a fellowship from the EMBL Interdisciplinary (EI3POD) programme under Marie Skłodowska-Curie Actions COFUND (grant agreement number 664726).



