Cryo-EM structures of human RNA polymerase I
Nature Structural & Molecular Biology 9 December 2021
10.1038/s41594-021-00693-4
EMBL researchers’ continued work with RNA polymerases provides fundamental information to understand enzyme mutations connected to rare diseases and cancers
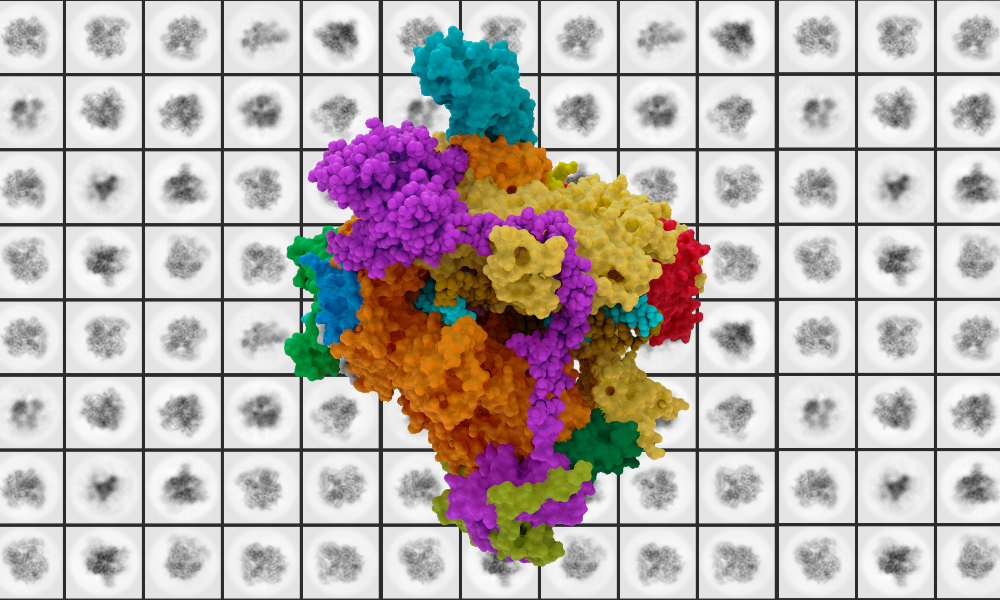
Using state-of-the-art technology and techniques, EMBL’s Müller group has provided the most detailed structure to date of human RNA polymerase I (Pol I), offering fundamental information about cellular function for those studying rare diseases and cancers. RNA Pol I has been implicated in certain disorders and cancers.
Their findings, published in Nature Structural and Molecular Biology, illuminate the structure and function of human Pol I in different functional states.
This marks the latest research chapter on this enzyme that began with prior Pol I research in yeast in 2013. It’s only fairly recently that the researchers moved from studying RNA Pol I in yeast to human Pol I.
In collaboration with Aleix Lafita, a computational biologist formerly at EMBL’s European Bioinformatics Institute (EMBL-EBI) in Hinxton, UK, the team was able to shed light on how this human version of the enzyme and some of its regulatory cofactors evolutionarily differ.
“When I started working on this project, we didn’t even know exactly how many subunits comprised human Pol I,” said Agata Misiaszek, the paper’s lead author.
In yeast, Pol I has 14 units, but in humans that number is just 13. As it turns out, most species have only 13 subunits to RNA Pol I – except for yeast and some other fungi – and the difference could be important.
“So, scientists have been studying an outlier for a long time,” said Mathias Girbig, one of the paper’s co-authors. “Interestingly, the place where this subunit is ‘missing’ is an important interaction site. The contrast to earlier work raises important questions that could help in understanding the regulation of Pol I’s function.”
Human RNA Pol I can only be obtained in small amounts, preventing the use of classical structural biology techniques, such as X-ray crystallography. However, with the advent of cryo-electron microscopy (cryo-EM) and CRISPR–Cas9 genome editing, EMBL researchers – along with others in the field – have begun making progress in understanding what Pol I looks like and how it works in human cells.
The group’s findings, along with their other recent work with human RNA polymerase III, complete the gallery of human RNA polymerases. This research lends insights into machineries that produce different types of RNA: from short ribosomal RNAs and transfer RNAs (Pol III) to long ribosomal RNAs (Pol I).
“Both Pol I and III are vital for the cell to function, but we now see some differences in key areas where mutations occur,” Misiaszek explained, “So, for example, in a rare disease like Treacher Collins syndrome, both polymerases are affected, but Pol I may be more so. Consequently, this begs the question of why this happens and what it means in patients.”
Nature Structural & Molecular Biology 9 December 2021
10.1038/s41594-021-00693-4