Get in touch!
If you have any questions or want to speak with one of our experts, please do get in touch. We would be delighted to hear from you. Contact us at: techdev@embl.org.
At EMBL, engineering and physics play a central role in driving technological innovation and research in the life sciences.
Engineering and physics are deeply embedded in the interdisciplinary research culture at EMBL. Experts from diverse technical fields – including biophysics, optics, spectroscopy, imaging, robotics, microengineering, and AI – work alongside biologists to develop new technologies and tools that enable breakthrough discoveries.
These disciplines provide enabling technology for many biological applications, such as studies on the cytoskeleton and cell mechanics, structural biology, microscopy, single-cell/single-molecule assays and high-throughput screening. They also allow us to embed AI deeply into lab workflows, optimising experimental design, automation, and hypothesis-driven research to streamline discovery.
If you have any questions or want to speak with one of our experts, please do get in touch. We would be delighted to hear from you. Contact us at: techdev@embl.org.

EMBL Grenoble technology teams provide a sneak peek into their latest collaborative project in structural biology services.
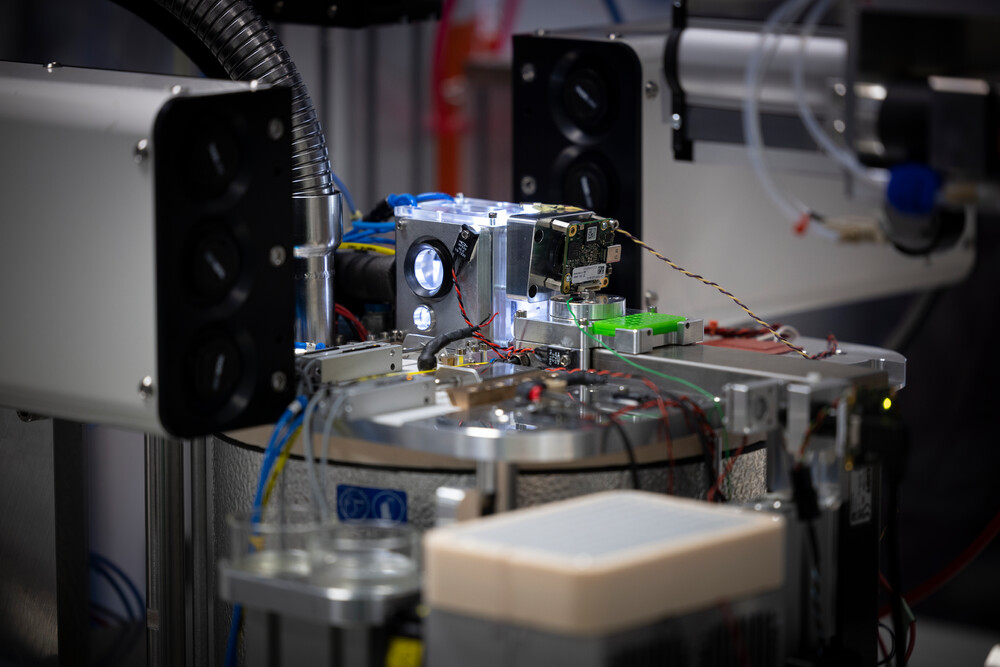
A platform for automated cryo-electron microscopy sample preparation and quality control.
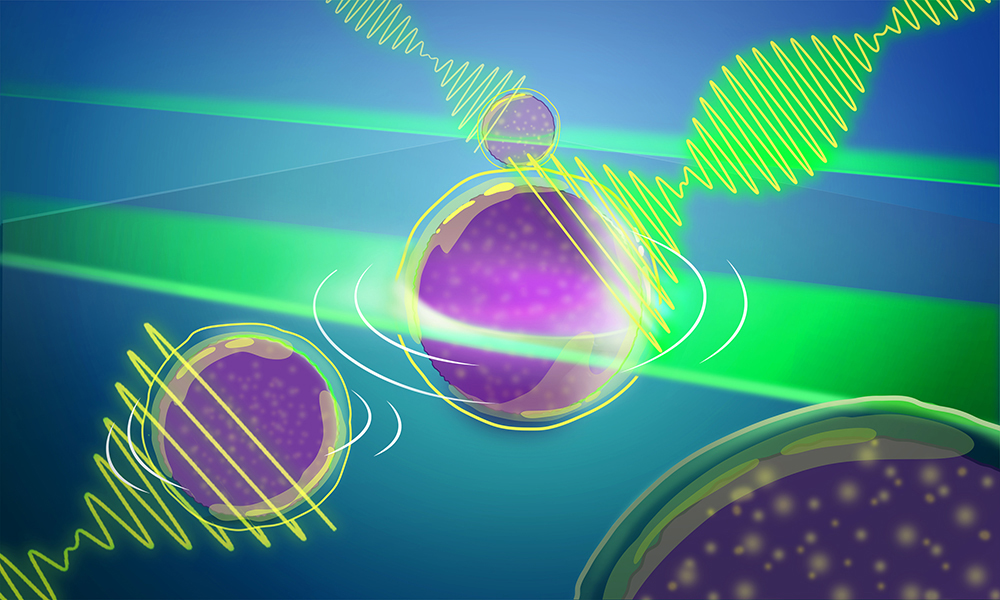
Another EMBL-engineered advance to Brillouin microscopy significantly widens the aperture and provides for quick, more efficient 3-D imaging of light-sensitive samples.

Researchers developed a microscope made to travel – miniature in scale, fast in imaging samples, and giant in resolution.
EMBL offers a unique opportunity to be involved in highly interdisciplinary projects in the life sciences, such as:
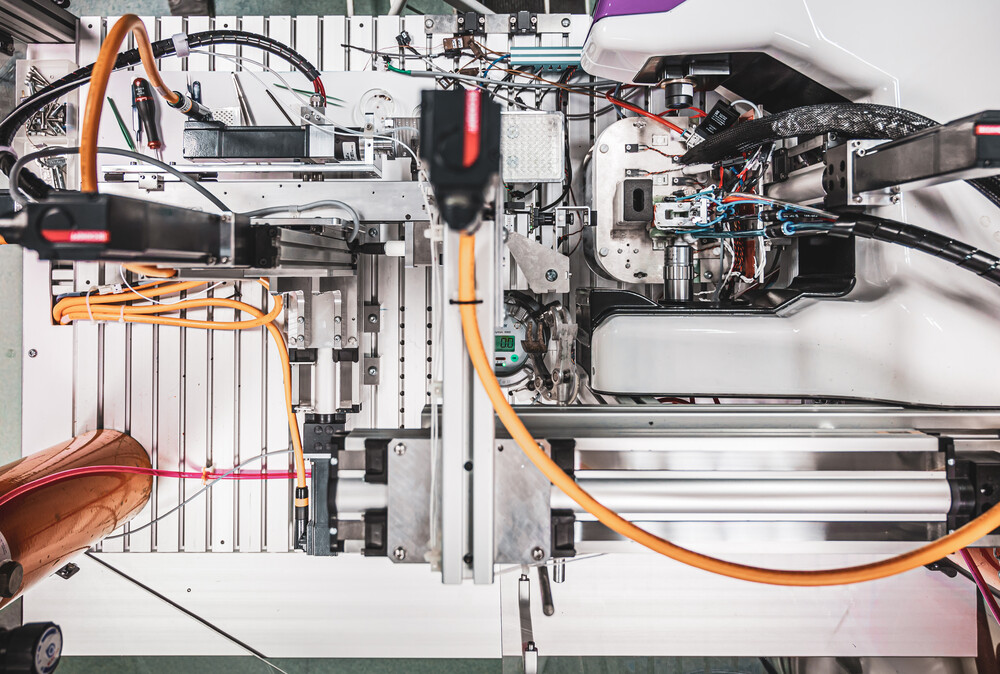
Papp Team, EMBL Grenoble
Designs and develops advanced instruments and methodologies for photon- and electron-based imaging, integrating high-precision mechanics, cryogenics, optics, electronics, and software.

Fiedler Team, EMBL Hamburg
Uses mechanical engineering, vacuum technology, X-ray optics, data acquisition, and control electronics and software to support X-ray experiments.

Prevedel Group, EMBL Heidelberg
Develops advanced, innovative optical imaging techniques to visualise cellular structure and function at high resolution in the native tissue context.
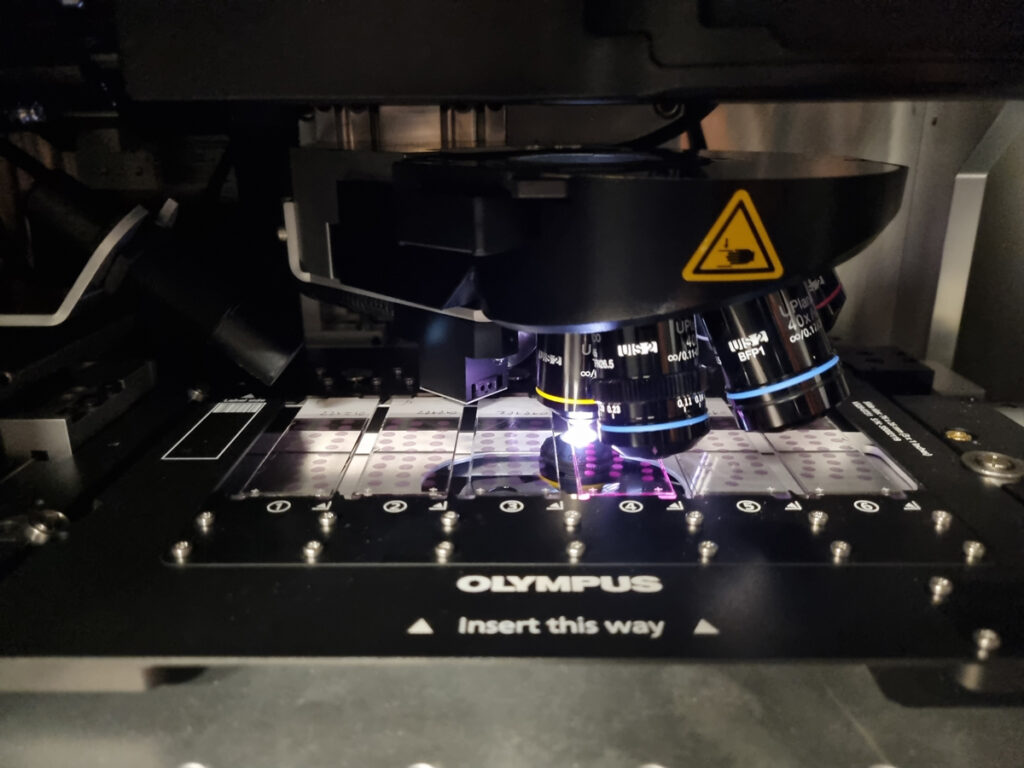
EMBL Rome
The facility integrates cutting-edge imaging technology, advanced image analysis, and automation with robotics and fluidics to support tissue biology and spatial-omics research.
Provides early-career students and researchers with an opportunity to gain hands-on experience in technology and tool development.
Offers interdisciplinary training that equips engineers and physicists with the skills to develop and apply cutting-edge technologies in the life sciences and helps them gain expertise at the interface of research and service provision.
Provides interdisciplinary training across scientific domains, allowing postdocs to apply their technological expertise to addressing complex biological questions and preparing them for leadership roles in collaborative, cross-domain research environments.
Introduces undergraduate students with non-biology backgrounds to the interdisciplinary world of current biology research.
Offers young researchers and engineers a journey into cutting-edge instrumentation, including detector design, radiation effects, data acquisition, AI, optics, and more. The school includes hands-on sessions and visits to research infrastructures.
The development of new instruments and technologies has a long history at EMBL. To ensure these developments benefit society, EMBL actively engages in technology transfer and industry relations.
We harness EMBL’s entrepreneurial spirit through technology transfer programmes and technology spin-offs.
EMBLEM is the commercial arm of EMBL, which identifies, protects, and commercialises the intellectual property developed at EMBL by EMBL alumni and by non-EMBL third parties.
AI at EMBL is EMBL’s ambitious initiative that aims to make the most of the vast potential of AI-based approaches to advance scientific discovery.
With support from more than 30 countries, laboratories at six sites across Europe and thousands of scientists and engineers working together, the European Molecular Biology Laboratory (EMBL) is a powerhouse of biological expertise. EMBL is an intergovernmental organisation, headquartered in Heidelberg, and was founded in 1974 with the mission of promoting molecular biology research in Europe, training young scientists, and developing new technologies.
EMBL currently employs more than 1800 people in Barcelona, Grenoble, Hamburg, Heidelberg, EMBL-EBI Hinxton (near Cambridge), and Rome.
Publishing hundreds of research articles and hosting dozens of conferences every year, EMBL is driving visionary fundamental research and training Europe’s future scientific talent.