
Read the latest Issue
As part of a series marking the tenth anniversary of the European Research Council, ERC grantee Stephen Cusack shares his vision for the next ten years
Many of us have experienced influenza – or ‘flu’ – as an illness with unpleasant symptoms like fever, coughing and sneezing, and an aching body, but usually we recover within a couple of weeks. However, certain strains of flu can be much more harmful – particularly in vulnerable groups like children, the elderly, and pregnant women – and around the world there are still many hundreds of thousands of flu-related deaths each year.
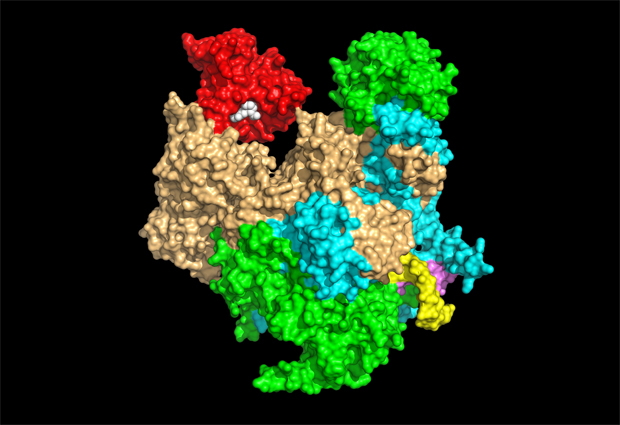
One way of tackling this problem is by developing anti-influenza drugs. A promising target for these drugs is one of the flu virus’s enzymes, influenza polymerase. My research group determined the molecular structure of this enzyme in 2014. It’s an assembly of three proteins that makes copies of the flu virus’s genetic material and also transcribes it – in other words, uses it to create strands of messenger RNA (mRNA), which tells a virus-infected cell to make viral proteins. To be recognized by the cell’s protein-making machinery, all mRNAs have to have a special chemical structure at the beginning known as the cap. The flu virus can’t make the cap itself, so instead it steals it from host cell mRNA.
We’ve been able to show how this works in three dimensions. The influenza polymerase enters the nucleus of an infected cell and binds to the human form of the polymerase enzyme, which makes human mRNA. It binds to the cap of a newly formed human mRNA strand and cuts out a short piece called a primer that contains the cap structure. The primer is then flipped into the active site of the viral polymerase to kick-start synthesis of viral mRNA. It’s an ingenious strategy known as ‘cap-snatching’, which is unique to the flu virus.
We’d like to know in more detail how influenza polymerase works. In the past we’ve mainly used X-ray crystallography to figure out protein structures, but you first need crystals of the protein you want to study, which can be difficult or in some cases impossible to obtain. It took several years before we succeeded in doing this with influenza polymerase. More recently, we’ve been able to make use of cryo-electron microscopy (cryo-EM), which involves rapidly freezing samples at very low temperature and imaging them using a beam of electrons, allowing us to observe detail at much smaller scales than is possible using a conventional light microscope. Since cryo-EM doesn’t require samples to be highly ordered – as in a crystal – we’re able to see individual influenza polymerase molecules in many different shape configurations in the same sample, allowing us to piece together the whole sequence of changes that the enzyme undergoes during its normal activity.
The structure of influenza polymerase is hard to determine using cryo-EM because it’s a small protein complex with flexible, moving parts. However, we’ve recently used cryo-EM to determine a detailed structure of it, comparable in quality to those obtained using X-ray crystallography. We’ve benefited from recent amazing developments in cryo-EM that have revolutionised the technique – the pioneers of these developments, including EMBL alumnus Jacques Dubochet, were awarded the Nobel Prize in Chemistry this year. Our continued cryo-EM studies on influenza polymerase will also benefit from the newly opened cryo-EM facility on the European Photon and Neutron science campus – where EMBL Grenoble is located.
Understanding the structure of influenza polymerase makes it easier to identify drug candidates that could stop it from working, halting the progress of the virus by preventing new viruses from being made. The parts of the influenza polymerase involved in cap-snatching, for instance, are prime targets for antiviral drugs. Many researchers and pharmaceutical companies are currently working on this problem, and I’m confident that in five years we’ll have potent anti-influenza drugs that target influenza polymerase.
We’ll also understand more about how the polymerase functions in the physiological environment of the cell. For example, how does it manage to sneak in and steal caps from strands of human mRNA, when these caps are usually protected by specialised proteins? To answer this and other questions, we’ll need not only the cryo-EM technique described above, but also cryo-electron tomography – an extension of cryo-EM that should make it possible to obtain three-dimensional images of influenza polymerase at work inside the nucleus of a host cell. These studies will bring many benefits, such as an improved understanding of the species barrier that usually prevents humans from being infected by strains of flu that infect other animals, such as birds. Occasionally this species barrier can be crossed – often with severe consequences. Understanding how that happens will be an important step toward preventing flu outbreaks and reducing the widespread health problems they cause.

The European Research Council (ERC) is a European funding body that offers substantial five-year grants to support researchers at all stages of their careers. Inspired by the ERC’s tenth anniversary in 2017, we asked some of EMBL’s ERC grantees to look ahead another ten years and share their vision for their field of research.
Looking for past print editions of EMBLetc.? Browse our archive, going back 20 years.
EMBLetc. archive