Uncovering how cells cover gaps
Scientists develop a clearer picture of dorsal closure and shed light on wound healing
Click on image to download High resolution .tiff
Researchers at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, came a step closer to understanding how cells close gaps not only during embryonic development but also during wound healing. Their study, published this week in the journal Cell, uncovers a fundamental misconception in the previous explanation for a developmental process called dorsal closure.
Scientists study dorsal closure, which occurs during the development of the fruit fly Drosophila melanogaster, to gain insights into wound healing in humans, as both processes involve closing a gap in the skin by stretching the surrounding epithelial cells over it.
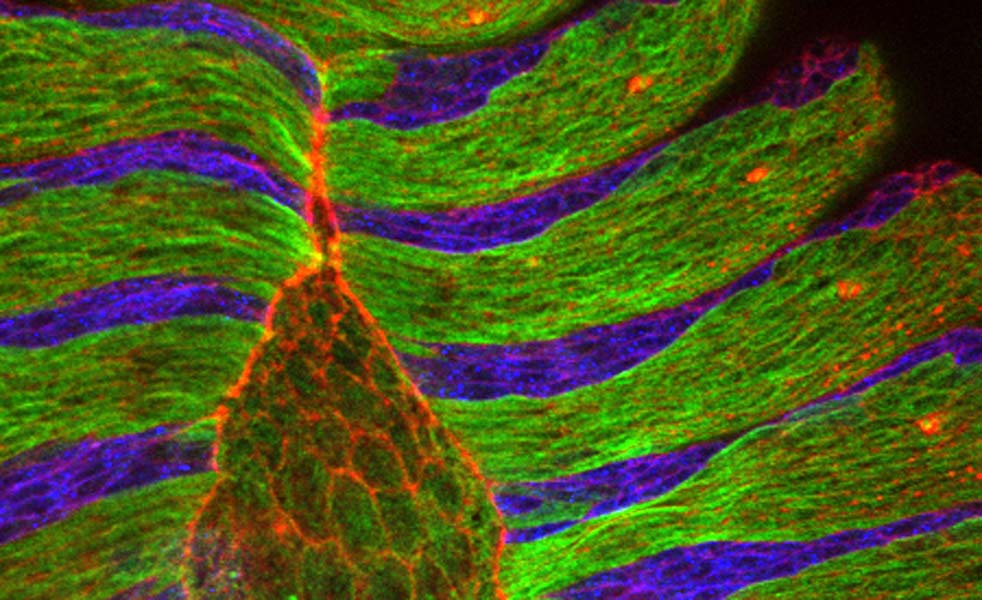
Dorsal closure involves three entities: the cells that fill the gap, called amnioserosa cells, a cable of the protein actin which runs around the gap, and the epithelial cells that eventually stretch over and seal the gap.Until now, scientists believed dorsal closure started when some unknown signal made the amnioserosa cells and the actin cable contract. The actin cable would then act like the drawstring on a purse together with the gradually contracting amnioserosa cells, it would pull the epithelial cells together until the gap was closed.
By taking more pictures per minute researchers in Damian Brunner’s group at EMBL improved the time resolution of the movies generally used to study this process, and made an important observation. They found that amnioserosa cells pulse throughout their life, constantly contracting and relaxing their surfaces.With each contraction they transiently pull on the surrounding epithelial cells, and then relax, letting them go.
By combining their movies with computer simulations, Aynur Kaya and Jerome Solon in Brunner’s group discovered that the actin cable doesn’t act as a drawstring, but rather as a ratchet. With every force pulse of the amnioserosa cells, the actin cable contracts and stops the epithelial cells from moving back away from the gap when the amnioserosa cells relax. This ratchetlike action means epithelial cells can move in only one direction: over the gap, bringing about dorsal closure. “Essentially, you have a field of cells that creates the driving force,” Damian summarises, “and then you need to translate this force into movement by adding ratchets that lock the cells into the state where they should move”.
The researchers believe this mechanism could apply not only to dorsal closure and wound healing, but also to many developing tissues, since moving tissue around is central to development.
Watch Movie: Dorsal closure close-up (.mov)
The pulsing amnioserosa cells (on the left) pull on the smaller neighbouring epidermis cells. The result is a stepwise dorsal-ward (towards the left) displacement of the epidermis front. Displacement is sustained by the ratchet-like function of the actin cable that forms at the boundary between amnioserosa cells and epidermis. The movie was recorded with a standard spinning disc confocal microscope using 60x magnification.
Watch Movie: Dorsal closure overview (.mov)
Clipping of a Drosophila embryo undergoing dorsal closure of the epidermis. The movie was recorded with a standard spinning disc confocal microscope using 60x magnification. An image stack was recoreded every minute.