Molecular basis of mRNA transport by a kinesin-1–atypical tropomyosin complex.
Genes & Development 17 June 2021
10.1101/gad.348443.121
EMBL scientists reveal the details of a cellular courier service that gets instructions from the DNA to where they need to be.
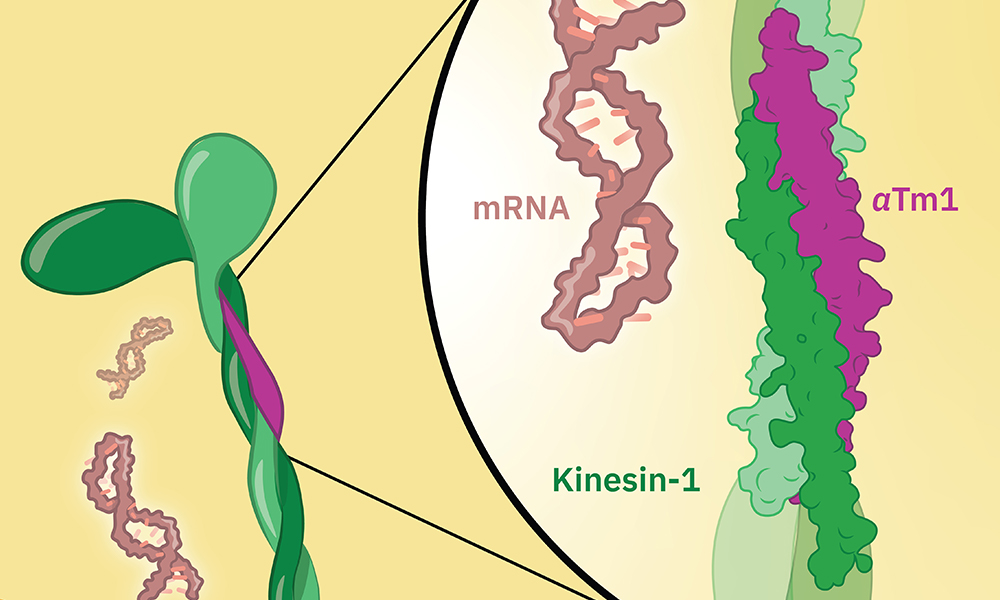
Protein production in cells starts in the nucleus, where an instruction-carrying molecule called a messenger RNA (mRNA) is created, using the cell’s DNA as a template. The mRNA moves out of the nucleus into the cytoplasm, where molecular machines use the instructions it carries to build a functional protein. The transport of molecules is often carried out by so-called motor proteins, which can move along protein filaments inside the cell called microtubules. One such motor protein is kinesin-1, which is known to transport cargoes over long distances on these microtubule tracks.
Often mRNAs are found in specific locations in the cell that are related to their function. An example of this is the oskar mRNA in fruit flies. In a developing fruit fly embryo, the oskar mRNA needs to be positioned at a specific location, where it defines the tail end of the embryo. The correct positioning of this mRNA is essential for the development and fertility of the future fly.
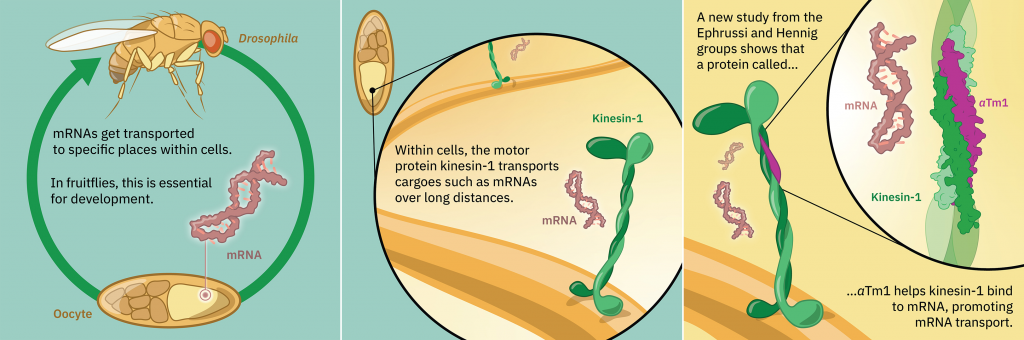
The oskar mRNA is transported by kinesin-1 inside cells, and this is known to involve another protein called atypical tropomyosin 1 (aTm1). Until now, however, it was not fully understood how these proteins cooperate and how aTm1 helps kinesin-1 to recognise its mRNA cargo.
A new study from the Ephrussi and Hennig groups at EMBL Heidelberg, in collaboration with the Löw Group at EMBL Hamburg, has generated a high-resolution crystal structure of the Kinesin-1/aTm1 transport complex in the fruit fly. The study reveals that aTm1 binds to a specific region in kinesin-1 and helps kinesin-1 bind to RNA, stabilising the transport complex.
Genes & Development 17 June 2021
10.1101/gad.348443.121
Looking for past print editions of EMBLetc.? Browse our archive, going back 20 years.
EMBLetc. archive