
Read the latest Issue
Scientists from the Wilmanns group have teamed up with experts across the Deutsches Elektronen-Synchrotron (DESY) research campus in Hamburg and at the SLAC National Accelerator Laboratory in California to show that naturally formed crystals can diffract X-rays. The first crystals successfully analysed with a free-electron laser inside the cells that produced them are unlikely to be the last.
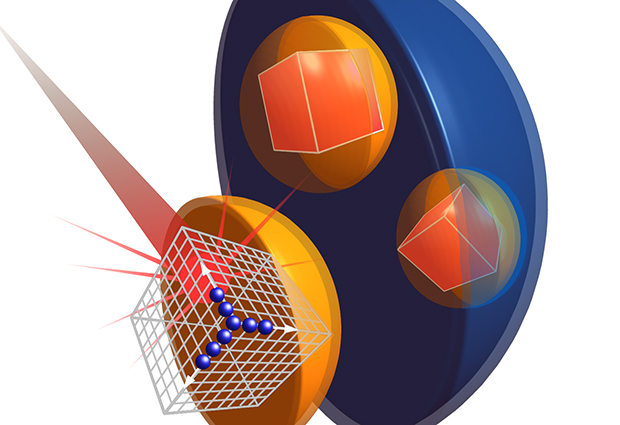
While structural biologists are familiar with the concept of growing protein crystals in the lab for X-ray crystallography experiments, many may not know that some organisms produce crystals naturally within their cells. “When we heard about these naturally forming crystals, we wondered whether we could use them for crystallography experiments,” says Daniel Passon, a postdoc in the Wilmanns group at EMBL Hamburg. “Producing protein crystals in the lab for crystallography experiments is not always easy – imagine if we could get cells to do this for us: a tiny crystal factory in a cell!”
It is a great example of the importance and potential of emerging infrastructures for the field of structural biology.
Crystallography uses X-rays to probe the 3D atomic structure of proteins that have been captured in their crystalline form, but the technique has its limitations. In a study published recently in the International Union of Crystallography Journal (IUCrJ), the team of Hamburg scientists instead used crystals grown inside yeast cells for crystallography experiments at the Linac Coherent Light Source, an X-ray free-electron laser facility. “This study would not have been possible without access to one of only two X-ray Free-Electron Lasers currently operational in the world,” says Matthias Wilmanns, Head of EMBL Hamburg, who oversaw the research, “It is a great example of the importance and potential of emerging infrastructures for the field of structural biology.”
The group studied crystals that occur naturally in parts of the cell called peroxisomes. These organelles break down large molecules such as fatty acids, keeping toxic processes safely within their bounds and away from the rest of the cell. In Hansenula polymorpha yeast cells, a protein called alcohol oxidase breaks down methanol molecules into useful byproducts.
To make effective use of the restricted space within the peroxisome, alcohol oxidase molecules are packed tightly into crystals; despite being so densely packed, the enzyme molecules inside this crystal are still active. “We think of crystals as rigid entities, but in fact they are not entirely solid,” explains Arjen Jakobi, a postdoc in the Wilmanns group and the Sachse group at EMBL Heidelberg, who carried out the work together with Passon and Wilmanns. “The methanol molecules pass through the crystals, reacting with the oxidase to become detoxified.”

While all of these yeast cells have peroxisomes, some cells have more peroxisomes than others, and peroxisome size varies from cell to cell, too. This natural variation posed a problem, as the researchers needed the crystals to be as large as possible, and as similar to each other as possible.
The Wilmanns group worked closely with colleagues at the University of Groningen who identified a mutant strain of yeast that only produces one large peroxisome per cell, each containing one large crystal. “Large is of course relative,” says Passon. At 0.001mm, the crystals were still too small for observations at even the most advanced synchrotrons, where they were likely to be combusted before data could be collected. “Free-Electron Lasers produce a large amount of photons in small bursts and have a very small parallel beam,” explains Wilmanns, “This makes them ideal for looking at such small crystals.”
The experiment was novel not only for EMBL’s scientists. “This field is its infancy and there are few leading experts worldwide,” says Wilmanns. “We teamed up with the Coherent Imaging Division at the neighbouring Center for Free Electron Laser Science (CFEL) at DESY and University of Hamburg, and benefited from the considerable experience and expertise of division Director, Henry Chapman and his group” he adds.
Having done some initial validation experiments on the beamlines in Hamburg, Wilmanns, Chapman and their teams set off to the Linac Coherent Light Source at SLAC with their precious peroxisomes. “For such a novel and exciting experiment, I was really keen to be there in person!” says Wilmanns. “It reminded me of being at the synchrotron 20 years ago – it is a very experimental set-up, but the SLAC staff are skilled and efficient.”

The group prepared two types of samples for the experiment: one with the peroxisomes inside their cells and the other with just the peroxisomes, removed from cells. “Surprisingly, we got better data when we measured the peroxisome inside the cell,” says Jakobi. “There was a lot less interference from the surrounding cell material than we expected.”
Surprisingly, we got better data when we measured the peroxisome inside the cell.
Having shown that it is possible to get data from crystals within a cell, the group now hopes to harness the natural ability of the peroxisome to produce crystals of other proteins, thereby side-stepping the need for laborious crystallisation experiments. “This could become a complementary method for structural biologists studying challenging proteins,” Wilmanns concludes.
Looking for past print editions of EMBLetc.? Browse our archive, going back 20 years.
EMBLetc. archive