International connections
During the development of the human brain, hundreds of billions of nerve cells in the nervous system are making new connections. Despite this unimaginable amount of wiring, the main component of our nervous system, the neuron, always seems to know exactly where to go, and extends nerve fibres – or axons – towards their final target with precision and determination.
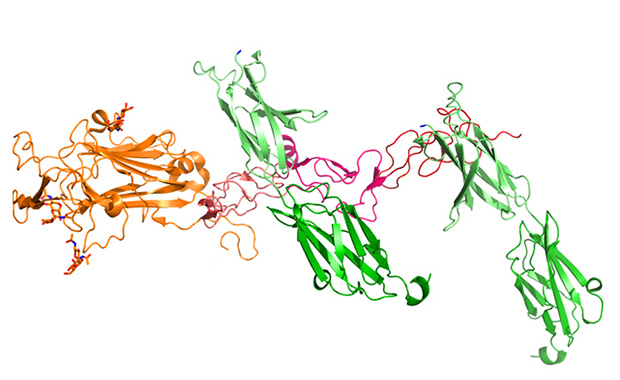
How is it possible that, in this seemingly random network of connections, nothing goes wrong? What invisible hand steers the neurons safely to their destination? Through a collaboration spanning several years, three continents and several close calls with airport security, Rob Meijers, group leader from EMBL Hamburg, and collaborators have determined the structure of an unusual protein complex that plays an important role in guiding neurons.
Triggered attractions
At the tip of growing axons sits a ‘growth cone’, a fan-shaped structure with finger-like extensions. Receptors expressed on the cone recognise signals from the cell’s surroundings, which they interpret into path-finding instructions, like a dog following a scent trail. These signals, or guidance cues, trigger an ‘attract’ or ‘repel’ response. One of the four major families of guidance cue proteins is netrin – from the Sanskrit word “Netr” meaning “one who guides” – a small but unique family of proteins that can trigger attraction and repulsion. How one molecule performs these contradictory push-and-pull functions has long been a mystery.
Netrins have undergone very few changes during evolution and are conserved across the entire animal kingdom. Meijers and colleagues studied netrin-1 in complex with one of its receptors, DCC, which together trigger an attraction. The structural data, published in Neuron, show that a single netrin-1 molecule can bind with two molecules of DCC simultaneously. “One binding site is specific to DCC,” explains Meijers, “but the second is not. It is a generic binding site that can also accept other receptors.” Exchanging DCC for another receptor in the non-specific site switches the neuron’s response from attraction to repulsion, changing the protruding axon’s direction of growth.

Boston-Hamburg-Beijing
The finding is the result of an ambitious research project that accrued many thousands of frequent-flyer miles for the scientists and their samples. “It has been an extraordinary journey,” says Lorenzo Finci, the postdoc who worked on the project with Meijers, and who globe-trotted between three of the world’s top science institutes – and into several nerve-wracking situations.
Finci was recruited by Jia-huai Wang from the Dana Farber Cancer Institute in Boston, to work in a newly established lab at Peking University. After receiving a short-term EMBO fellowship to fund a three-month stay in Germany, he arrived in Hamburg in November: “My first Thanksgiving outside of the US,” he says. “I didn’t know where to find a traditional Thanksgiving turkey, so instead I celebrated with a burger, which I’d heard were famous in Hamburg!” During a cold winter, Lorenzo worked together with Nina Krüger from Rob’s group to work out how to purify netrin and, crucially, how to put it together with DCC.
Straight to the beam
Once Finci’s fellowship ended and he returned to China, the project became even more international, which proved as stimulating as it was stressful. “Everything works on a 24 hour cycle,” says Meijers, “When I wake up, China has already been working for six hours. By the time I go to bed, Boston has another six hours worth of data to hand back to Beijing.” Transporting samples was also problematic: “Getting liquid samples in and out of China is impossible by mail.” Finci took advantage of a family trip to Turkey, enlisting his cousins to race him through Istanbul traffic so that he could ship samples to Hamburg overnight.
Getting liquid samples in and out of China is impossible by mail.
Meijers and his group got to work, and within a week crystals had formed – but was it the sought after DCC-netrin complex? “I received an excited email that it was,” Finci recalls. “I called my supervisor, Jia-huai Wang, maybe 50 times before he finally called me back and I shared the good news!” Meijers stresses the advantage of having immediate access to the beamlines and sample characterisation facilities at the PETRA III synchrotron ring on the Deutsches Elektronen-Synchrotron (DESY) campus. “We crystallised the protein complex using our on-site facility the same day. Normally, you sacrifice a few crystals before finding the ideal conditions, but those crystals went straight to the beamline and the first one diffracted successfully”.

Binding site negotiations
Later that week, the group noticed there were actually two binding sites on netrin, and decided to have a friendly bet on which site was more physiologically relevant. “Site 1 would be celebrated with Dutch-brewed Heineken, site 2 with Tsingtao,” says Finci.
Characterising the two binding sites meant more trips for Finci. “He acted as a ‘mule’, travelling back and forth between Beijing and Hamburg 10 times,” says Meijers. “After one 13-hour trip from Beijing to Hamburg, the customs agent called me over,” says Finci. “I declared the bag’s contents, and showed them the paperwork. Two hours later, I had an audience and was being investigated by five agents and two dogs!” EMBL Hamburg’s administrators came to the rescue, rushing over new paperwork with official EMBL seals. On returning to Beijing, this time with netrin in hand, the same thing happened. “I learned two important lessons: to carry documentation with impressive seals, and a box of chocolates for my friends the customs agents.”
Most protein binding sites have a ‘lock and key’ mechanism – this second non-specific binding site is unusual
It turned out that both binding sites are important, so rather than beer, the teams celebrated with wine and a Peking Duck dinner when Meijers visited Beijing. “Most protein binding sites have a ‘lock and key’ mechanism,” explains Meijers. “This second non-specific binding site is unusual: it is positively charged, as are the receptors, so normally they would repel each other, like equivalent magnet poles.” Instead, it seems that sulphate ions sit in the binding site and negotiate which receptor is received. “These negatively charged ions are organised in such a way that they can also be replaced by certain sugar-like molecules, called heparan sulphates,” adds Meijers. “We know that deactivating sugar molecules confuses neuronal wiring – to make a link that suggests sugars and small molecules are important in selecting receptors could be relevant for rational drug design.”
Beyond neurobiology
Although their work has concentrated on the field of neurobiology, the results have possible applications in the field of cancer biology. Many cancer cells produce netrin to attract blood vessels that nourish them and help them grow. Interrupting this netrin supply could starve the tumour, or at least prevent it from growing. DCC stands for ’Deleted in Colorectal Cancer‘ and its absence seems to result in uncontrolled cell growth and tumour metastasis. “Maybe now that we know more about how DCC and netrin-1 work, we can attempt to influence cell growth and stop tumour metastasis in its tracks,” says Meijers.



