
X-rays reveal surprising shape of scaffolding protein
The shape of PDZK1, an important scaffolding protein in cells, is full of surprises
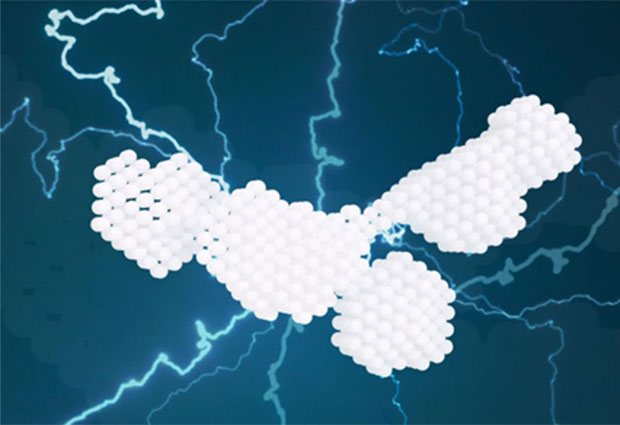
While bioinformatics tools had suggested that the four domains of PDZK1 would behave like beads on a string, moving around in a highly flexible manner, X-ray experiments now show that PDZK1 actually has a relatively well-defined L-shaped conformation with only moderate flexibility.
This discovery was made by a research team led by EMBL group leaders Dmitri Svergun and Christian Löw – who is also a group leader at the Centre for Structural Systems Biology (CSSB) in Hamburg, Germany. The journal Structure published their results on 13 September 2018.
Scaffolding proteins like PDZK1 mediate interactions between proteins on the membranes of human cells. Löw, Svergun and colleagues performed small-angle X-ray scattering (SAXS) studies at DESY’s X-ray light source PETRA III to obtain the new information on the shapes and arrangement of PDKZK1’s domains, as well as on their interactions.


