New electron microscopy images reveal the assembly of HIV
EMBL researchers provide the as yet closest look at the structure of immature HIV
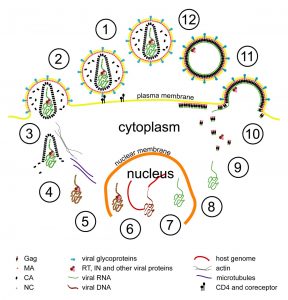
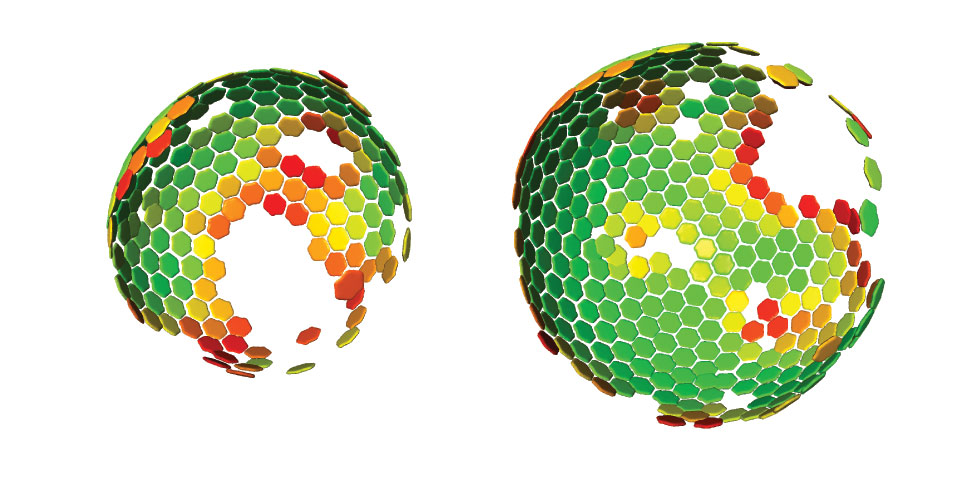
Scientists at the European Molecular Biology Laboratory (EMBL) and the University Clinic Heidelberg, Germany, have produced a three-dimensional reconstruction of HIV (Human Immunodeficiency Virus), which shows the structure of the immature form of the virus at unprecedented detail. Immature HIV is a precursor of the infectious virus, which can cause AIDS. The study, published in the 22-26 June online edition of PNAS, describes how the protein coat that packages the virus’ genetic material assembles in human cells. Drugs that block this assembly process and prevent the virus from maturing into its infectious form are considered a promising therapeutic approach. HIV consists of an RNA molecule that carries the genetic information of the virus and is surrounded by protective protein and membrane layers. During infection the virus deposits its genetic material into a human cell where it reprogrammes the host cell machinery to generate many copies of the viral genome and initiates the production of a viral protein called Gag. In the immature virus, many copies of Gag interact to form a roughly spherical lattice that encloses the virus’ genetic material.The virus then leaves the cell with the help of proteins of the host and infects new cells.
Using a method called cryoelectron tomography researchers in the groups of John Briggs at EMBL and Hans-Georg Kräusslich at the University Clinic Heidelberg generated the as yet highest resolution 3D computer reconstruction images of the immature Gag lattice. The results suggest a simple model of HIV formation in human cells: multiple Gag proteins interact to form a hexameric lattice that grows with an inherent curvature and that incorporates new proteins stochastically. Several further steps in which Gag is cleaved by an enzyme are necessary to transform this immature lattice into its mature, infectious form.
Briggs and his team are now working on producing an even higher resolution structure of the protein lattice to gain a more detailed understanding of the virus’ assembly and maturation processes, which may eventually help to find weak points that could be targeted by drugs.
Cryoelectron tomography is a technique with which a sample is instantly frozen in its natural state and then examined with an electron microscope. Images are taken from different directions and assembled into an accurate 3D reconstruction by a computer.