Binding bracelet
Scientists pin down the function of Vasa, a protein that is critical for keeping DNA healthy and viable from one generation to the next.
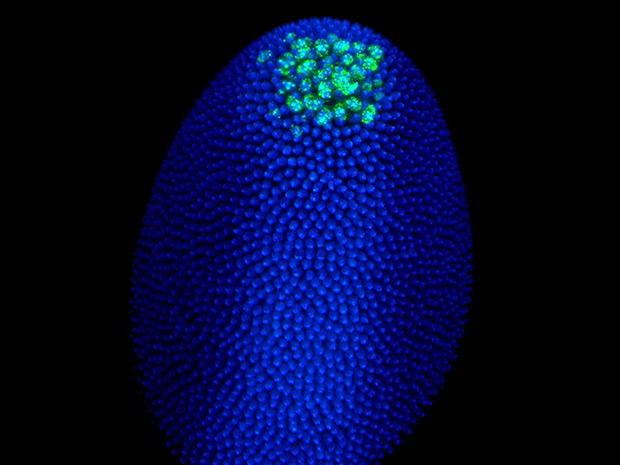
Just as many people find it hard to sit still for long periods of time, DNA doesn’t just wait patiently in its double helix form to be read and duplicated. A large proportion of an organism’s genome is composed of transposons – segments of DNA that can jump in and out of chromosomes. Many are dormant – inactive genetic fossils from our evolutionary past. But some can still be activated and have the potential to severely disrupt normal gene function. Keeping those transposons in check is crucial, especially in egg and sperm cells, where the viability of the future generation is at stake.
To protect their precious genes from transposon interference, reproductive cells have a kind of miniature immune system that finds and destroys the RNA that is transcribed from transposon DNA. Special molecules called piRNAs identify the RNA sequences and enable an enzyme (called piwi in fruit flies, miwi in mice and hiwi in humans) to cut them in two. But rather than digesting both transposon fragments, one fragment is preserved and used as a template to generate new piRNAs to hunt down the target sequence. It’s an elegant strategy in the ongoing war against transposons. “Every time an enemy is killed, the enemy becomes food for new defenders, and these new defenders are made by this cycle,” says Ramesh Pillai of EMBL Grenoble. “It’s like a trophy-taking, let’s say; a little piece of the enemy, which in this case is a fragment to make a piRNA.”
This process is so vital to reproduction that it’s been conserved across all animals, even sea life. “This is a remarkable thing, because in mice if you block it, there’s no sperm produced. If you block it in flies, in the ovaries, the flies can’t lay eggs or the eggs that are laid are infertile. If we don’t have it, we will not be fertile,” says Pietro Spinelli, a PhD student involved in the project.
Every time an enemy is killed, the enemy becomes food for new defenders.
For such an important cycle, its exact mechanics remain shrouded in mystery. One key player is the protein Vasa, which has long been used to identify germline cells – the cells that will become eggs and sperm – and is suspected to help protect DNA in those cells. Its exact purpose, however, has eluded scientists because whenever Vasa is extracted and analysed, no associating proteins have been found. A collaboration between Pillai’s molecular cell biology lab and head of EMBL Grenoble Stephen Cusack’s crystallography lab allowed the two groups to ‘freeze’ Vasa (with any interaction partners still attached) by inducing a mutation that prevented it from changing shape, and then analyse its structure and any other associated proteins.
Jordi Xiol in Pillai’s lab found that Vasa protects the template transposon fragment from being digested by fastening around it like a bracelet. Vasa also binds a collection of other ‘handoff’ proteins that are attached to piwi, “so as soon as the transposon is cut, a fragment can be easily handed to piwi because piwi is already assembled on Vasa on the transposon,” explains Xiol.
In a collaboration with Anne Ephrussi’s lab in Heidelberg, the scientists experimentally blocked Vasa in cells in living fruit flies’ ovaries. The flies’ offspring failed to mature into larvae and were always infertile, confirming the protein’s crucial role in healthy development.
This is definitely a project that can happen only in a place like EMBL.
Pillai and his team now have their sights set on purifying each of the proteins that binds to Vasa individually, so they can determine the structure of the whole assembly. “We know that Vasa functions as a construction site for different proteins. But how does it do it? Where does it assemble? We don’t know,” says Pillai.
Further collaborations with crystallography labs will be essential for answering those questions, because they move beyond the capabilities of molecular biology and demand a level of detail that is only attainable with highly specialised techniques. “Grenoble is the right place to do this kind of work because we can determine at atomic resolution how Vasa does its job,” Spinelli explains. “That’s a beautiful thing to see.”
The group is also interested in studying Vasa and its partners in other animals, to ultimately determine how it might function in humans. “My lab is now collaborating with Dónal O’Carroll’s group at EMBL Monterotondo to introduce the mutation in the mouse Vasa. This is definitely a project that can happen only in a place like EMBL, where collaboration is very easy,” says Pillai.