The Steinmetz Cardiomyopathy Fund was established in memory of Dr. Michael Steinmetz, a pioneer in biotechnology and healthcare who died of an aggressive, inherited form of dilated cardiomyopathy (DCM).
Familial DCM is a genetic disease that occurs when the heart muscle dilates, cannot contract normally, and therefore cannot pump blood efficiently. It is one of the main causes of heart failure and the leading reason for heart transplantations. Our mission is to find a cure for this deadly disease and help all affected families, as currently there are no targeted treatments. The funds will be used for collaborative research led by Dr. Lars Steinmetz, who is a Senior Scientist in the Genome Biology Unit at EMBL, Professor of Genetics at Stanford University, and Co-Director of the Stanford Genome Technology Center.
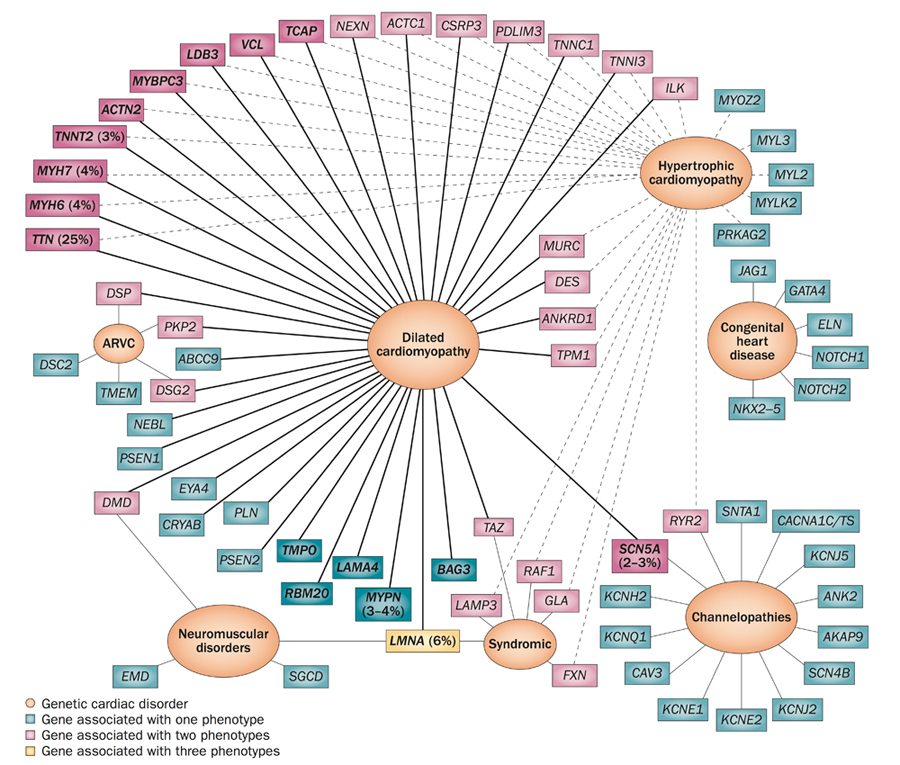
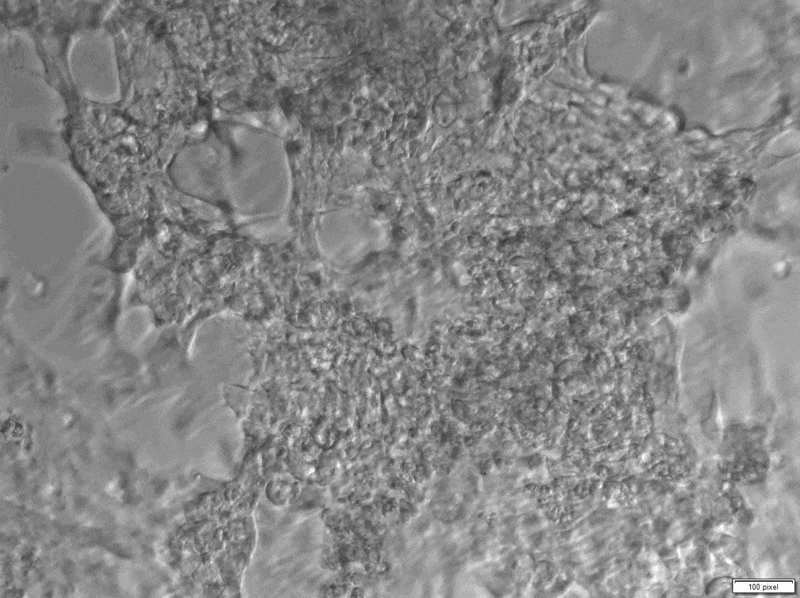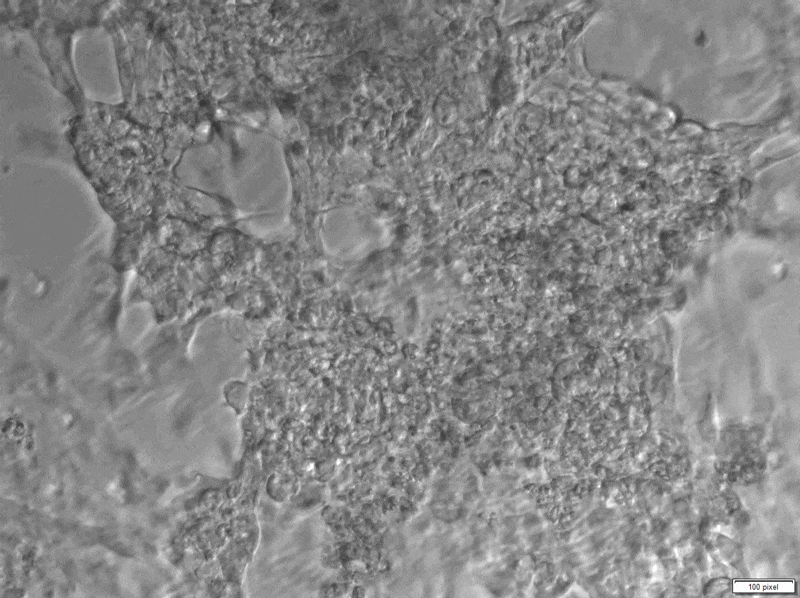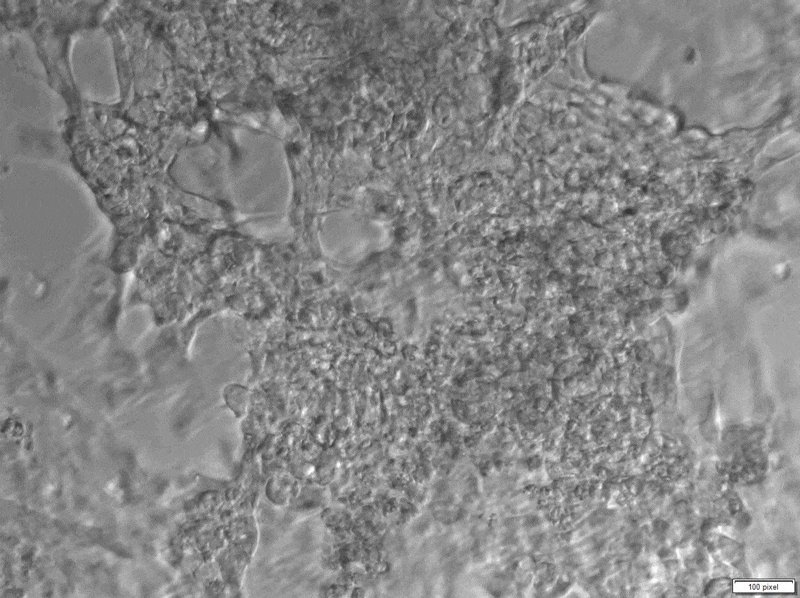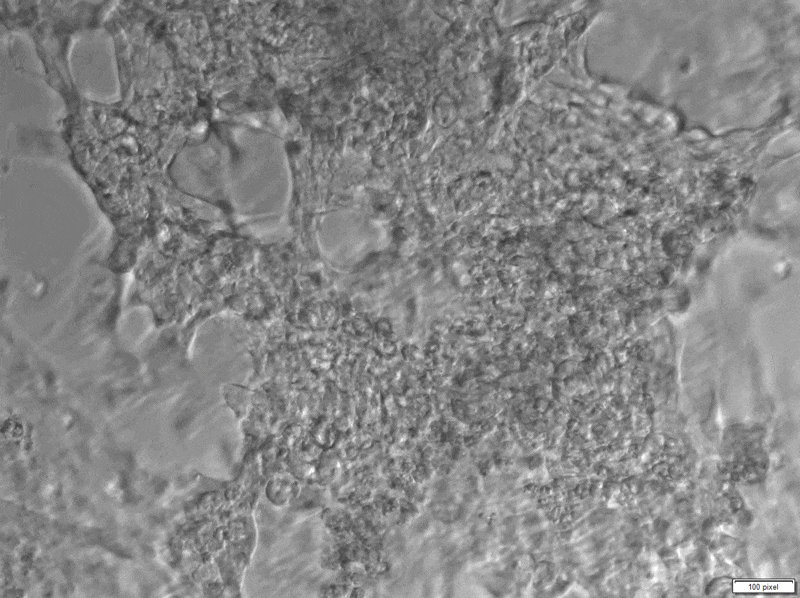
Much progress has been made in understanding the genetics of cardiovascular diseases like DCM (Figure 1), but developing targeted therapies will require a better understanding of how genetics affects heart function. There is clear evidence that a gene called RBM20 plays a key role in familial DCM. RBM20 encodes a protein that helps process (“splice”) RNA molecules in the heart, and mutations in this gene are responsible for this disease in an estimated 3% of all cases. We are now using diverse strategies to understand the exact mechanisms by which RBM20 causes DCM and design new therapeutic approaches. To understand the clinical impact of RBM20 mutations, we have generated induced pluripotent stem cells (iPSCs) from adult patients and healthy controls. These cells can be differentiated into various human tissues, including heart cells (cardiomyocytes). Using the newest sequencing technologies and cutting-edge experimental systems we are studying how mutations in RBM20 affect the characteristics and functionality of such cardiomyocytes. In addition, we are using genome editing approaches to introduce disease-causing mutations into healthy cells so that we can observe their consequences in a well-studied genetic background. Using the patient heart cells we have engineered in the lab (Figure 2), we have set up a platform to test drugs that may counteract the harmful effects of RBM20 mutations.
Currently, diagnosis and management of DCM rely heavily on complex medical procedures including echocardiography, heart biopsies and genetic testing. Major challenges lie in establishing less cumbersome systems to monitor disease progression and determine early hallmarks of disease. Your donation to the Steinmetz Cardiomyopathy Fund will be used to dissect the molecular mechanisms underlying DCM and accelerate the development of new personalised approaches to detect, monitor and ultimately treat the disease.
We would be very grateful for gifts to support this work, large or small. These gifts will help us make important discoveries that could improve the lives of millions of DCM patients.
Contact us to find out more