The giant protein titin helps build muscles
Imagine grabbing two snakes by the tail so that they can’t wriggle off in opposite directions. Scientists at the Hamburg Outstation of the European Molecular Biology Laboratory (EMBL) and collaborators from King’s College in London have now discovered that something similar happens to a protein that is crucial in the formation of muscle tissue. Their work appears in the current issue of the journal Nature.
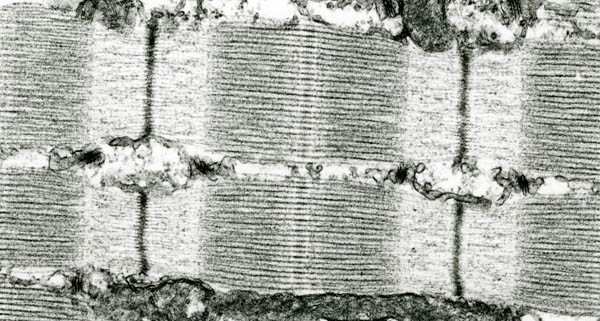
Under the microscope, muscle looks like millions of tiny pistons, stacked end-to-end into long rows. These structures, called sarcomeres, permit the contraction and relaxation of muscle that allow our bodies to move. Sarcomeres are connected at the ends by Z-disks, thick bands of densely-packed molecules.
“Sarcomeres are very complex structures, and for many years we’ve been investigating the steps by which they are formed,” says Matthias Wilmanns, Head of the EMBL Hamburg Outstation. “That probably starts when proteins link up to each other in very big assemblies. The meeting point is the Z-disk, but unraveling the connections has been difficult.”
Wilmanns’ lab and the team of Mathias Gautel, an EMBL alumnus and now at King’s College, have thought that a molecule called titin, the largest protein made by human cells, is involved. Titin is anchored in the Z-disk and is so long that it spans half the length of a sarcomere. Its size and position – putting it into contact with all the major components of sarcomeres – suggests that it might help in their assembly.
The latest study, which arises from more than a decade of collaborative research by the Wilmanns and Gautel teams, gives a solution to how this might happen. Peijian Zou and Nikos Pinotsis from the Wilmanns lab obtained crystals of parts of the titin molecule bound to another protein, called telethonin. They analysed the crystals on high-energy X-ray beamlines at EMBL’s Hamburg station, on the site of the German Electron Synchrotron Radiation Facility (DESY). From the extremely detailed image of the connections between the proteins they discovered that telethonin links the tails in a unique way that may give some clues to sarcomere assembly.
The Gautel lab used advanced microscope techniques to watch the molecules link to each other in live cells. “We knew that telethonin acted as a sort of ‘cap’ or ‘bolt’ at the end of the titin molecule,” Gautel says. “What we couldn’t see is how it connected two separate copies of titin together. That’s what this study has shown.”
Wilmanns adds: “We discovered that telethonin has a kind of internal symmetry that lets it grasp two titin molecules running off in opposite directions. That’s new. We’ve discovered other single proteins that can link to DNA molecules in ‘palindromes’, but this is the first time we have seen proteins themselves linked like this.”
Other molecules enter the Z-disk from sarcomeres on both sides; it is likely, the scientists think, that some of the connections follow the example of titin and telethonin. The groups will now look for other examples. The terminal assembly complex investigated in this work, though, only covers a tiny part of the giant muscle protein titin, which possesses tens of thousands of amino acids in some isoforms. “There are probably hundreds or even thousands of more interactions down the road. We have found one of the first and eagerly anticipate discovering many others, probably coming with lots of surprises,” Wilmanns concludes. “We have just started to get insight into one of the most complex systems in the human body.”
“This work is a nice example of how modern biology combines approaches from cell biophysics and structural biology, and it gives us a way to discover why some mutations in titin are linked to diseases,” says Gautel.



