The Data Use Ontology to streamline responsible access to human biomedical datasets
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100028
The GA4GH Data Use Ontology (DUO) supports a data authorisation and access framework to streamline consent to use biomedical data

A large group of international experts within the Global Alliance for Genomics and Health (GA4GH), including users at EMBL’s European Bioinformatics Institute (EMBL-EBI) and the Broad Institute of MIT and Harvard, have developed the Data Use Ontology (DUO) standard. DUO annotations allow terms and definitions in data consent forms and data sharing policies to be standardised and machine readable. This helps in streamlining consent to use controlled-access biomedical datasets.
Human biomedical datasets often contain sensitive information about individuals. Consequently, the owner of the data must take great care when these data are being made available for secondary use by other researchers. Access and sharing of such data need to comply with legal, ethical, and informed consent rules and regulations. Navigating this complex landscape can slow research down as researchers require permission to access sensitive datasets.
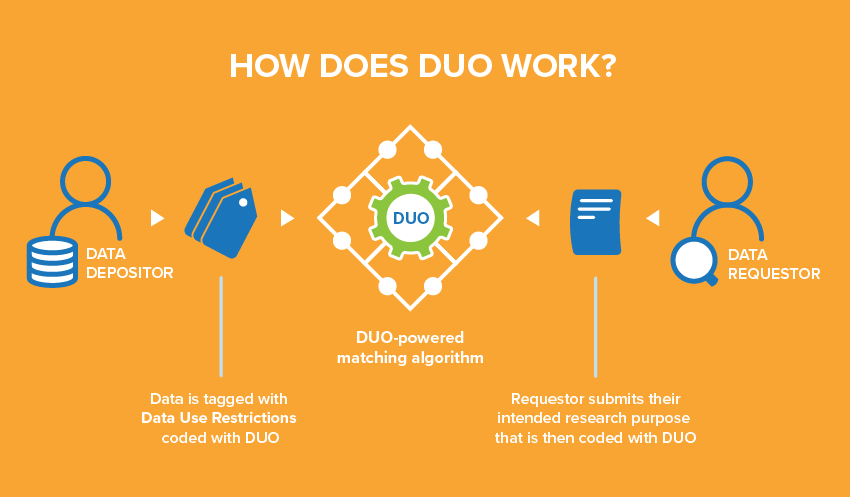
Using the Data Use Ontology (DUO) standard will streamline these processes by supporting a data authorisation and access framework for granting researchers permission to reuse biomedical datasets based on their credentials and research purposes. As described in a recent publication in a special issue of Cell Genomics, DUO, together with the GA4GH Passport standard, accelerates responsible sharing of biomedical datasets worldwide.
To retrieve and reuse biomedical datasets, researchers must submit a data access request to Data Access Committees. This can be a long process as a typical workflow involves manual review of applications against the data use letter that specifies how the dataset can be used, to determine whether access should be granted.
“Research consent forms often do not provide clear information about biomedical dataset sharing. Not having a standard way of representing data use conditions can really slow research down when trying to re-use those datasets,” said Melanie Courtot, Metadata Standards Coordinator at EMBL-EBI and co-lead of the DUO development team. “Using the GA4GH DUO standard is a first step to help researchers around the globe access the biomedical data they need quickly and easily.”
“I’m incredibly thankful to the many contributors to DUO,” added Melanie. “This was a huge collaborative effort that received input from many international experts and users.”
DUO is a machine-readable standard vocabulary of data use terms, aiming at describing data use conditions and automating the matching of specific datasets against the data access requests. Pairing DUO with the GA4GH Passport further automates this step by providing a researcher’s authentication and authorisation levels.
“Over 200,000 datasets worldwide have already been annotated with DUO terms and it has been successfully implemented in various institutions such as the Broad Institute and the Wellcome Sanger Institute,” said Tommi Nyrönen, Head of Node at ELIXIR Finland, and co-lead of the GA4GH Data Use and Researcher Identities Work Stream that developed the DUO. “There’s still more to be done; we want to include different data types and the Resource Entitlement Management System tool we are developing and deploying with EGA will leverage both DUO and Passport implementation.”
This work was published as part of a Cell Genomics special issue focusing on the work of GA4GH. To find out more, see the list of articles below.
This post was originally published on EMBL-EBI News
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100028
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100029
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100032
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100030
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100031
Cell Genomics 10 November 2021
10.1016/j.xgen.2021.100027
Looking for past print editions of EMBLetc.? Browse our archive, going back 20 years.
EMBLetc. archive