Read the latest Issue
How cells have got molecules surrounded
Ground-breaking microscopy techniques have enabled scientists at EMBL Heidelberg to shed new light on how cells perform endocytosis – a function that is key to many cellular processes, such as ingesting nutrients and cell-signalling.
The process of endocytosis generates bubble-like membrane vesicles that surround the molecules to be ingested and move them from the cell surface into the cell. In this study, published in Developmental Cell, a cross-disciplinary team from five research groups at EMBL and the European XFEL demonstrates the significance of a particular type of proteins, called clathrin adaptor proteins, to the process.
Scientists have known about the existence of clathrin adaptor proteins, and their role in linking clathrin proteins to the cell membrane, for several years. The clathrin and adaptor proteins coat a patch of the membrane that is pulled inwards, forming a pocket around the molecules that are being ingested. The structure then detaches, with the molecules inside, to become a protein-coated vesicle inside the cell. The traditional theory explains that this process is mostly induced by the clathrin.
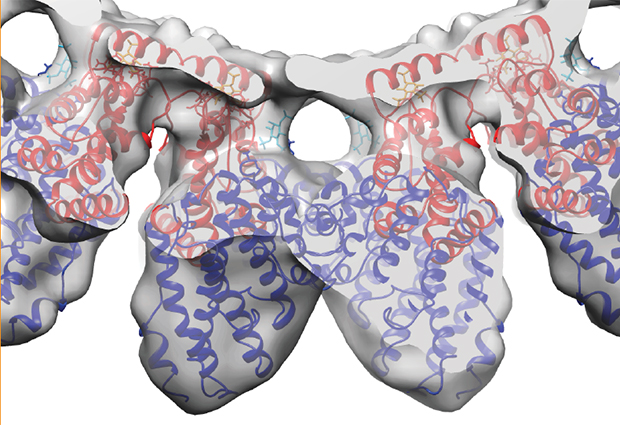
Using cryo-Electron Microscopy, the researchers examined two different clathrin adaptor proteins: Ent1 and Sla2. They discovered that, when mixed together, they form their own complex lattice structure on the surface of the membrane, thus suggesting they actually have a very important role to play in organising the vesicle coat and, crucially, for enabling the membrane to bend inwards.

Based on the cryo-EM structures, the team built a quasi-atomic model of the adaptor organisation on the membrane. They further tested this new theory by selectively mutating genes to impair the formation of the organised adaptor structure in cells and found that it prevented endocytosis.
“The existing view was that the clathrin cage alone was sufficient to organise the proteins on the vesicle coat, but the reality is much more complicated than that and we’ve shown the importance of these clathrin adaptors to the process,” explains Carsten Sachse at EMBL-Heidelberg.
It’s a complicated challenge because we are taking snapshots of a dynamic process involving extremely small components.
“Our imaging and genetic techniques enabled us to really see what was happening inside the cell,” says Marko Kaksonen, at EMBL-Heidelberg. “It’s a complicated challenge because we are taking snapshots of a dynamic process involving extremely small components.”
Around 50 different proteins are involved in endocytosis and the team now plans to use their new technique to look at the others and to gain an even more sophisticated understanding of this complex and important mechanism.