CRISPR: from clipping scissors to word processor
New platform transforms CRISPR gene editor into precision tool
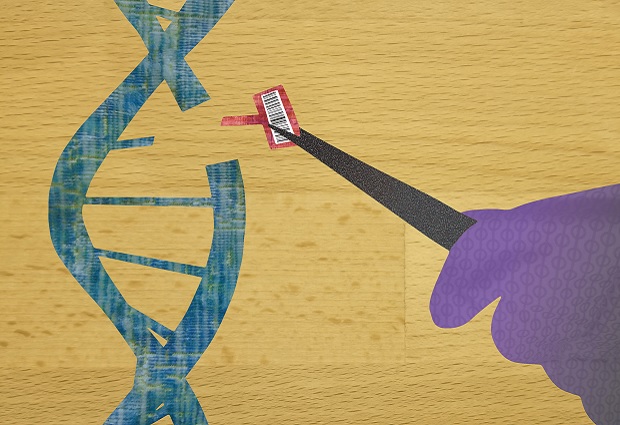
Using the gene-editing tool CRISPR to snip at DNA is often akin to using scissors to edit a newspaper article. You can cut out words, but it’s difficult to remove individual letters or instantly know how the cuts affect the meaning of the text. Someday, CRISPR could be used to ‘clip’ disease-causing genetic mutations in patients. But for such precision medicine to become a reality, the precision of the tool CRISPR still needs to improve.
In work that will help to make the gene editing process more precise, EMBL scientists, together with researchers at Stanford University, the Joint Institute of Metrology and Biology (JIMB), Texas A&M University, and Brandeis University, have developed a new kind of CRISPR platform called MAGESTIC. The new platform allows to operate CRISPR less like a blunt cutting tool and more like a word processor by enabling an efficient ‘search and replace’ function for genetic material. Nature Biotechnology publishes the results on 7 May 2018.
“We are reaching a state where we have not only achieved the ability to sequence the order of base pairs in genomes but we can also make changes to them. We still need a better understanding of the consequences of our edits,” says Lars Steinmetz, EMBL group leader and Stanford University Professor, who is the senior author on the paper. “With MAGESTIC it’s like being able to make small edits to individual letters in a book, and being able to see what effect it has on the meaning of the text. Our method also allows the new piece of information to be placed at exactly the right page where the cut occurred.”
Gene editing entails targeting the CRISPR machinery to cut at desired locations throughout the genome, and directing the cells to introduce designed edits at the cut sites by providing a ‘donor’ DNA, which the cell’s repair machinery uses to replace the original sequence. Searching for a suitable ‘donor’ DNA is an enormous challenge for the cell, as millions to billions of base pairs have to be combed to find the correct ‘donor’ DNA. MAGESTIC aids the cell in this search by artificially recruiting the designed donor DNA directly to the cut site in a process termed “active donor recruitment.” Such recruitment caused a seven-fold increase in precision editing efficiency. MAGESTIC also introduces a new kind of cellular barcode to track designed mutations in each cell. The new feature allows for the integration of a barcode into chromosomes, which makes it stable, and easy to find and count later on.
“With MAGESTIC, we can make precise and targeted changes to genotypes”, says Sibylle Vonesch, one of the first authors of the paper and a postdoctoral fellow in EMBL’s Steinmetz group. “For example, we can introduce small, isolated alterations into yeast genomes and investigate their effects. Eventually, we hope to be able to predict what happens in a cell based on its genetic variant.”
This is a modified version of the original press release by NiST.