
Fish on fire
While immune sensing mechanisms vary widely between animals, ASC specks – particles that play a key role in triggering inflammation – form in humans as well as zebrafish. In a study published in The Journal of Cell Biology, Paola Kuri and Maria Leptin show how this process happens in zebrafish in real time
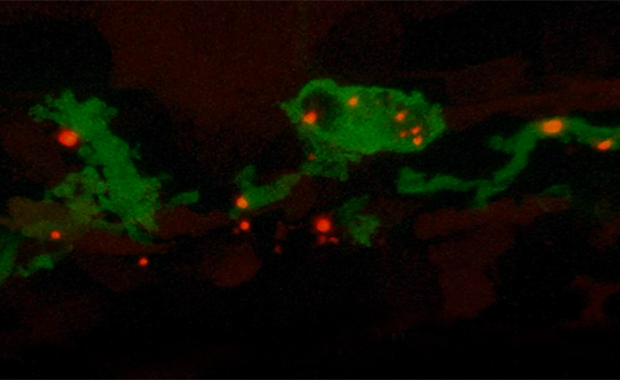
Walk us through what is happening in this video clip of ASC assembly

Kuri: Inflammasomes are big multi-protein complexes that assemble when specialised molecules inside the cell detect damage. ASC is a protein that links these molecules with the next step in signalling by aggregating into a clump, which is called an ASC speck because of the way it appears under the microscope.
To explore the effects of speck formation in the live fish, we inserted a copy of ASC into the zebrafish genome – one that enabled us to induce ASC expression, trigger speck formation in different cell types and study the consequences. Because we’ve tagged ASC with a fluorescent protein, we can visualise this live throughout the entire organism.
The process of speck assembly is basically a snowball effect – all of the available ASC protein in the cell clusters into the same huge aggregate. In this video of zebrafish skin, the ASC specks are red. Each cell forms a single speck, to which other proteins are recruited to initiate reactions that we show will ultimately result in cell death of skin cells. By labelling macrophages in green, we can visualise how these cellular garbage collectors come and clean up the cellular debris.
Nicole Schieber, who is part of Yannick Schwab’s team at EMBL, helped us look at the structure of specks using correlative light and electron microscopy. By combining images from light microscopy and electron microscopy, she was able to show what a speck formed in vivo [in a living organism] looked like in astonishingly fine detail, which confirmed and extended predictions that were made in vitro [in an artificial environment outside of the organism].

How else does your paper build on previous research?
Leptin: To visualise ASC inside a cell, previous cell culture experiments introduced an additional copy of the gene – in which a fluorescent label was attached to ASC – into the cell’s genome. This means ASC can be seen, but it will be expressed at levels not originally determined by the genome. When we put proteins in cells and play around with them, that’s not normal! Experiments looking at ASC speck formation in cells with naturally occurring levels of ASC have only been done in dead, stained cells. The advantage of our system is that we can visualise speck formation in real time with ASC levels that are found normally in the fish.
The advantage of our system is that we can visualise speck formation in real time
Kuri: We achieved this by using CRISPR-Cas9, a powerful gene editing technology, to modify the zebrafish genome. With CRISPR, we can avoid introducing extra copies of the gene because we’re able to modify the genome itself at specific spots. Here we seamlessly inserted a fluorescent label into the endogenous ASC gene. This means ASC is produced at normal levels with the fluorescent label attached. And you can see it live in the entire fish!
How far would visualising ASC speck formation have applications for human health?
Leptin: There is a huge interface between inflammation and health. Inflammation, on one hand, is good. It alerts the body to danger and sends in professional cells to deal with it. On the other hand, it’s what makes you feel bad, and it can go seriously wrong! Allergies and many diseases such as Alzheimer’s are linked to inflammation when it is not kept in check and becomes chronic, which has also been linked to higher ASC speck numbers. Inflammatory systems have to be poised so that they quickly turn on when there is real danger, yet do not turn on spontaneously.
Kuri: Inflammation is not just limited to immune cells – it can happen in tissues and cells that are not normally linked to the innate immune system. ASC is everywhere inside zebrafish, including the gut, gills and brain, but I was particularly interested in the skin cells of the animal, which come into contact with the outside world. This is why looking at an entire organism becomes relevant. Shedding light on how inflammation works in these tissues contributes to our understanding of how and why inflammation can go wrong.


