Cracking a virus protection shield
Scientists reveal the structure of a protein that packages the viral genome and helps viruses to replicate while avoiding human immune reactions
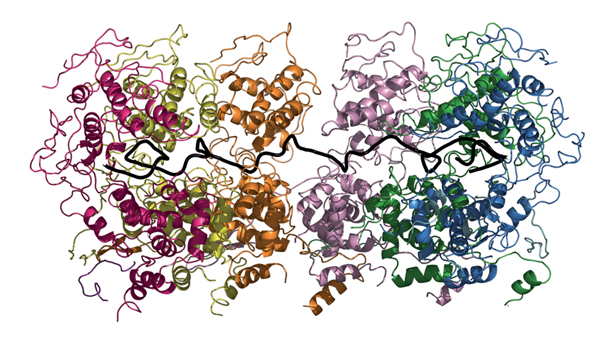
Ebola, measles and rabies are serious threats to public health in developing countries. Despite different symptoms all of the diseases are caused by the same class of viruses that unlike most other living beings carry their genetic information on a single RNA molecule instead of a double strand of DNA. Now researchers from the Institut de Virologie Moléculaire et Structurale (IVMS) and the Outstation of the European Molecular Biology Laboratory (EMBL) in Grenoble have obtained a detailed structural picture of a protein that allows the rabies virus to withstand the human immune response and survive and replicate in our cells. The study that is published in this week’s online edition of Science suggests new potential drug targets in rabies and sheds light on how similar approaches can help fighting other viral diseases.
When the rabies virus enters a human cell through the membrane, the RNA molecule that carries its genes is transported into the centre of the cell. Here it redirects the cellular machinery of the host to produce many new copies of the virus that go on to infect more cells. One molecule that is crucial in this process is a viral protein called nucleoprotein. The protein ensures that on its way through the cell the virus RNA is not destroyed by the immune response of the host.
“Nucleoprotein is vital for the rabies virus,” says Rob Ruigrok, Head of the IVMS. “It is one of the few proteins that the virus brings into the host cell and it wraps around the RNA like a protection shield. Without this shield the RNA would be degraded by the enzymes of the human immune system that try to eliminate the invader.”
To investigate how exactly this protection shield works, Aurélie Albertini from Ruigrok’s team obtained crystals of nucleoprotein bound to RNA. Examining the crystals with high-intensity X-ray sources at the European Synchrotron Radiation Facility (ESRF), Amy Wernimont from Winfried Weissenhorn’s group at EMBL Grenoble produced a high-resolution image of the protein.
“Nucleoprotein acts like a clamp,” says Weissenhorn. “It consists of two domains that like two jaws clasp around the RNA strand. Many nucleoproteins bind side-by-side along the length of an RNA molecule and make it inaccessible for degrading enzymes but also for the machinery needed to replicate the virus. This means that the protection shield must be flexible and able to distinguish between different types of enzymes trying to gain access.”
The detailed structural picture suggests that upon a signal a part of the protein located between the two main domains might act as a hinge that moves the upper jaw out of the way when time for replication has come.
“This dynamic mechanism makes nucleoproteins an excellent drug target,” says Ruigrok, “Small agents that bind to the protein in such a way to block its flexibility and keep it in the closed state, would prevent replication of the virus and would stop it from spreading.”
Rabies virus shares this protection strategy with other viruses of its class; in Ebola, measles and Borna virus similar complexes of RNA and nucleoproteins have been found.
“This means that our results do not only have implications for the design of new drugs against rabies, but they suggest new therapeutic approaches in a variety of diseases, some of which are much more threatening than rabies. On a different note, the conservation of the nucleoprotein system also leaves room for evolutionary speculations about common ancestors and primordial infectious units of RNA viruses,” Weissenhorn concludes.