
Remodelling the cell
Cells, like people, come in all shapes and sizes. To get to their final shape they have to make important structural changes in the membranes that form their borders.
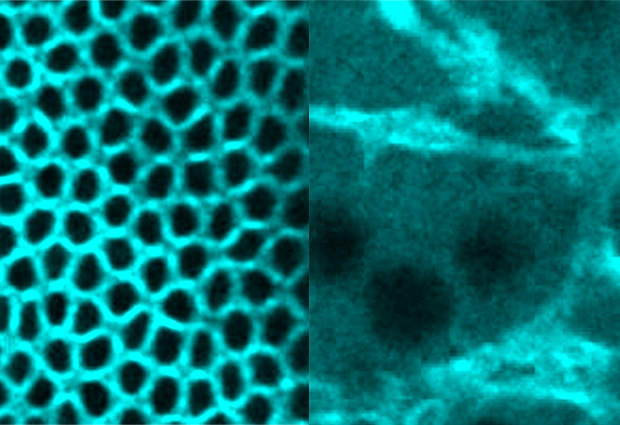
As cells get bigger, they obviously need to create more membrane, but sculpting the cell into a defined shape also requires tightening and contracting parts of the membrane too. Coordinating these processes is essential for cells to acquire their correct form. New research from Stefano De Renzis’ group at EMBL Heidelberg has begun to show how this is achieved, as reported in a paper published this week in the Journal of Cell Biology. Stefano’s group studies the way cells change shape as embryos grow and develop, and the signals cells rely on to make sure they change shape appropriately. In recent experiments, Stefano and colleagues looked at how the embryo of the fruit fly Drosophila goes from being a ‘syncitium’ — a giant cell with many nuclei — to a collection of 6000 individual cells, each with one nucleus. “During this process, the surface area of the embryo’s cell membranes increases 30-fold, as membranes grow around each nucleus to define a new cell,” says Stefano. This entails expanding the membrane in some places, and tightening it in others to give the cells their shape — and coordinating these two processes. Using tools developed by Carsten Schultz’s lab (see factbox), Alessandra Reversi, postdoctoral fellow in the De Renzis group, found that this is achieved by altering the ratios of the different building blocks of cell membranes, in particular two kinds of molecules: phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol (3,4,5)-triphosphate (PIP3). High levels of PIP2 relative to PIP3 lead to contraction of the membrane; by contrast, high levels of PIP3 prevent this contraction and allow the membrane to expand. PIP molecules do not themselves cause contraction of the membrane, however. Instead, networks of actomoysin, which are made up of actin and myosin proteins and are attached to the cell membrane, do this hard work.
“We found that PIP3, along with another protein called bottleneck, inhibits the assembly of actomyosin, which prevents contraction of the membrane,” says Stefano. Although the precise details of how this is achieved are not known, Stefano thinks that bottleneck binds to actin and cause it to cross-link, preventing myosin joining the complex. Likewise, PIP3 may recruit other proteins that bind to actin with the same effect. The next step for Stefano and colleagues is to identify the upstream signals affecting PIP levels. It’s already known that proteins called PIP kinases and PIP phosphatases are crucial in determining the ratios of PIP2 and PIP3. “So the regulators of these proteins are the key molecules to focus on,” says Stefano.
From talk to tool
The questions scientists want to answer usually demand having the right tools at hand. If the requisite tools are not available in a researcher’s own lab, collaboration becomes essential. The recent research on phospholipid ratios and the regulation of membrane growth is a case in point. Stefano De Renzis’ lab is focused on unravelling the cellular dynamics and signalling processes involved in morphogenesis, during the development of Drosophila embryos. Carsten Schultz’s lab is frequently developing tools for studying signalling pathways, especially those involving lipids. After hearing a talk by Stefano at EMBL, Carsten realised that his tools would be ideal for tackling the processes Stefano studies, and the collaboration was born. And once the group leaders had decided to work together, their energetic postdocs – Alessandra Reversi and Devaraj Subramanian – took over, says Carsten. “They really grabbed the ball and ran with it.”


