The first step in generating recombinant baculoviruses using the transposition-based method is cloning the gene of interest in a suitable transfer vector containing the Tn7L and Tn7R sites for transposition into the bacmid DNA. Vectors compatible with this system are for example the commercially available pFastBac vectors, the MultiBac acceptor vectors pACEBac1 and pACEBac2, the biGBac vectors pLIB, pBIG1 and pBIG2 and the MacroBac vectors.
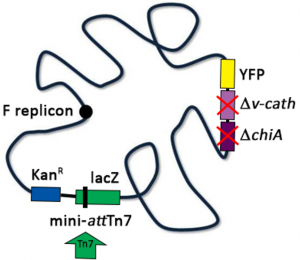
In a second step, this construct is transformed into competent E. coli cells carrying the bacmid DNA. In our lab we use E. coli DH10EMBacY cells for this step in the process, as they have the benefit of having the v-cath cysteine protease and the chiA chitinase genes deleted, which significantly reduces the viral proteolytic activity and the cell lysis. If the transposition of the gene of interest into the bacmid DNA is successful, the lacZa gene will be interrupted, which allows blue/white screening on agar plates containing X-GAL to identify positive clones. The recombinant bacmid DNA can then be isolated from positive (white) clones and used for transfection of Sf9 (or Sf21) cells. There are many different commercially available transfection reagents (e.g. FuGENE, X-tremeGENE, TransIT, …) that can be used for this step.
After amplification of the baculovirus particles, larger insect cell cultures can be infected for the recombinant protein expression. If desired, the viral titer can be determined by a plaque assay or via flow cytometry using anti-GP67 antibodies.
Small scale expression and purification tests can be performed by doing a time course with different virus-to-cell ratios (e.g. 1:100, 1:500, 1:1000 etc.). As the DH10EMBacY bacmid DNA also contains the YFP gene under control of the polH promoter, virus performance and protein production can be monitored by measuring the YFP fluorescence as well. Once the optimal time frame and virus-to-cell ratio have been determined, the expression cultures can be scaled up for the large scale protein purification.