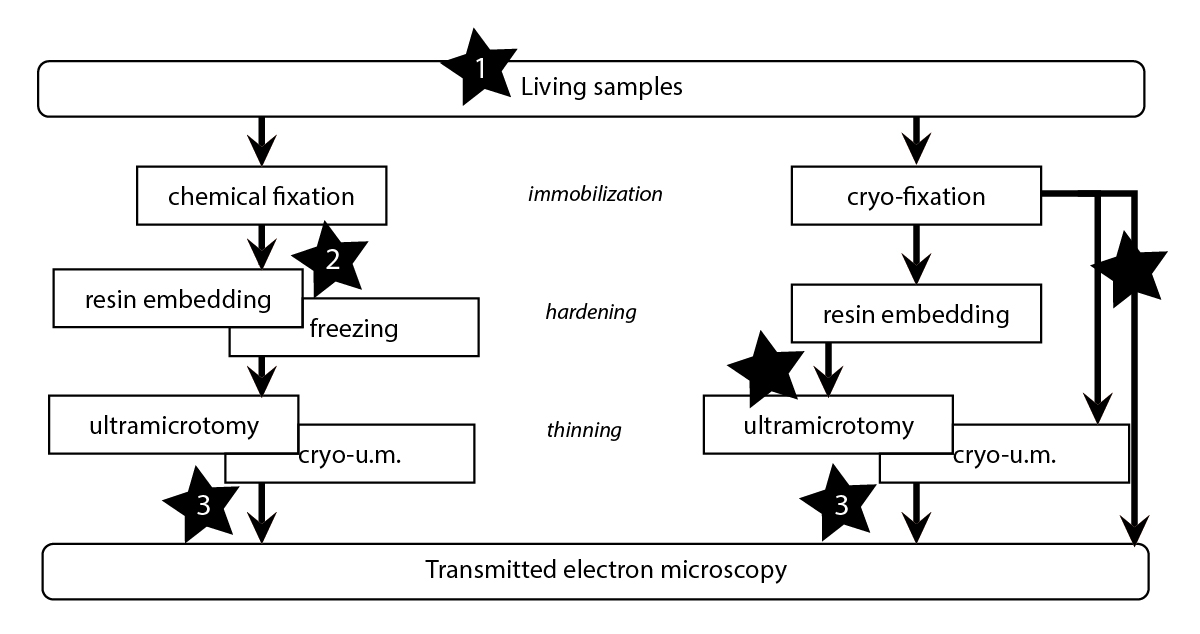
CLEM techniques allow the analysis of the same specimen with a combination of light and electron microscopy tools. Very powerful, they allow the selection of rare objects or events in complex, heterogeneous samples. Unfortunately, because of their tedious implementation and need of interdisciplinary skills, such techniques are almost solely utilized by the laboratories that developed them. Over the past few years, the EMCF has chosen to offer CLEM as a service to its users community, and is now becoming a reference site for it in the field. This implementation has been successful because the staff of the facility has contributed closely to the development of the technology and was able to bring it toward the necessary versatility for an efficient usage in a facility environment. This was performed via strong collaborations with research groups and teams (Antony, Pepperkok, Briggs, Kaksonen, Ellenberg, and Schwab) and with the Advanced Light Microscopy Facility.
A very nice illustration of the efficient relations between research groups and the EMCF is the methodological transfer performed after the groundbreaking development done by the Briggs and Kaksonen groups on correlating fluorescence microscopy and EM tomography on resin sections (Kukulski et al, 2011). This method, that could be qualified as post-embedding correlation, has achieved an unprecedented precision (<100nm) for the localization of fluorescent protein expression at the ultrastructural level. It relies on defined sample preparation protocols, on the use of bi-functional fiducial markers and on a computer assisted targeting of the features of interest. With the help of both groups, this technique is now accessible in the EMCF. Facility members can perform the experiments autonomously and are now able to train the users. In 2015, we have organized a first workshop in order to spread the method throughout the scientific community. This successful experience was repeated in April 2016 and in 2017.
You can find a detailed step-by-step tutorial on the method in EMBL’s e-learning channel.
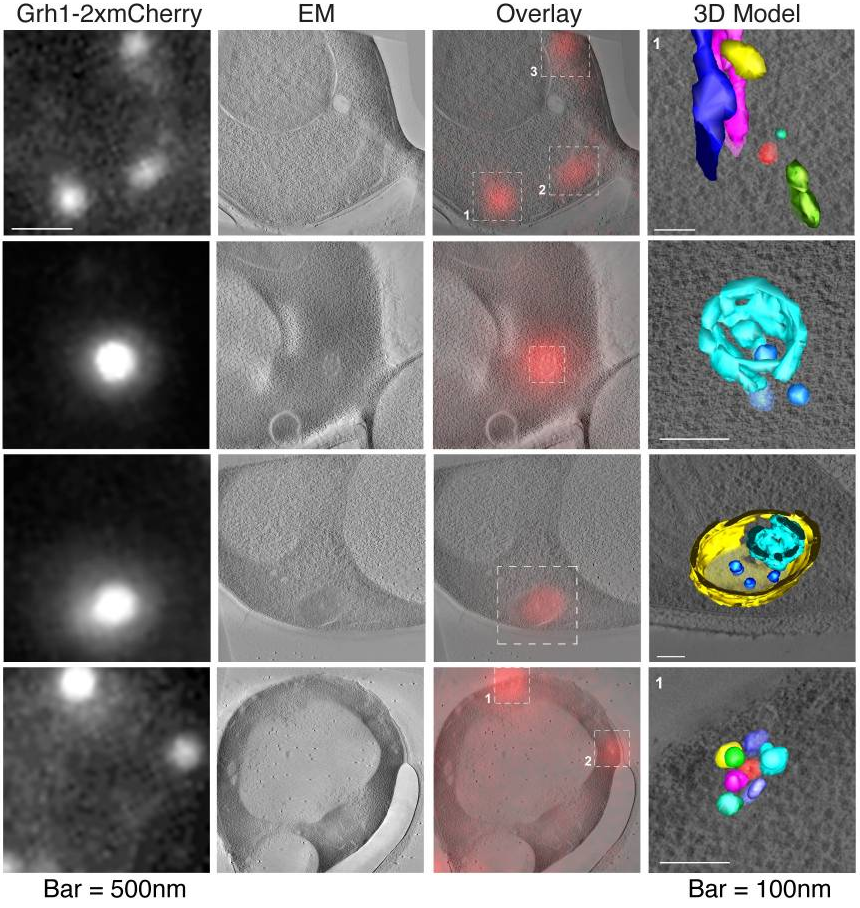
From Curwin et al. Elife 2016
When interested to correlate live imaging on cultured cells with EM, a large range of solutions is offered to the experimenters. They can rely on the use of laser etched landmarks at the surface of the culture substrate (Tängemo C et al, 2011), or on the implementation of a system of coordinates on the substrate before seeding the cells to them, either by shadow casting a carbon pattern, or by laser etching (Spiegelhalter et al, 2010, 2013, Romero-Brey et al, 2012). Live imaging can also be performed on bulk specimens (nematodes, zebrafish embryos, murine tissues). It can then be correlated with EM by using targeted approaches (Karreman et al, 2014, 2016). It is to be noted that these techniques are still being further developed, to reach a high degree of precision and throughput. This work is carried out by the Schwab team in close collaboration with other groups/team and facilities at EMBL. Once fully successfully developed they will all be offered as Facility services.