Matthieu Boulard
Group Leader
ORCID: 0000-0001-5135-8529
EditEpigenetic gene silencing in mammals

Group Leader
ORCID: 0000-0001-5135-8529
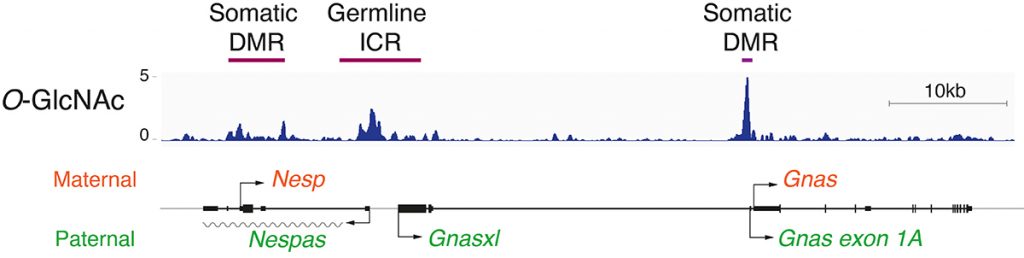
EditWe aim to understand the mechanisms by which mammals functionally partition their genomes. Commonly referred to as epigenetic silencing, this biological process ensures the stable silencing of retrotransposon promoters and restricts the expression of a defined set of genes to one of the two available alleles. The latter are so-called “mono-allelically expressed” and include imprinted genes, genes on the inactive X-chromosome, genes encoding olfactory receptors, as well as immunoglobulins and T-cell receptor loci. Epigenetically repressed promoters are set apart from typical silent genes by the nature of their transcriptional silencing, which is maintained even in the presence of the trans-acting factors sufficient to activate their expression. This phenomenon is most evident in the case of mono-allelically expressed genes, whereby the two homologous copies display opposite transcriptional states while being in the same nucleus.
Our team strives to understand how chromatin-based mechanisms repress these specific set of genes during embryonic development.
We will continue exploring the molecular mechanisms that sustain epigenetic gene regulation, as well as the cellular readout of cytosine methylation. We address these fundamental biological questions in vivo using the mouse as a mammalian model organism. Our experimental approach combines genetics, genomics, transcriptomics, and microscopy.