Focus on: Insulin
Overview
This teaching resource will guide you through consecutive steps to explore the biological structure of the insulin molecule – from primary to quaternary structure.
How to start:
- Select the level of protein structure which you would like to work on via the tabs below.
- Inform yourself about the structural details of insulin and follow the exercises.
- Try to answer the questions in the Insulin quiz.
- The Insulin worksheet can be printed out and worked on to test yourself on the structural biology of insulin. Color the structures and explore them in 3D!
Info
Insulin is initially synthesized as preproinsulin, a 110 amino acid polypeptide that contains additional sequences:
- A “pre” amino-terminal sequence (signal peptide of 24 amino acids), which enables the secretion of the protein.
- A central “pro” sequence (the C peptide of 35 amino acids) which determines the correct folding of the protein.
After preproinsulin is translated in the endoplasmic reticulum, an enzyme cuts off the 24 amino-terminal amino acids, leaving proinsulin, which in turn folds and allows the formation of the disulphide bonds between cysteine residues.
At this stage, the protein passes into the Golgi apparatus, where the C peptide is removed, forming mature insulin, which is then stored in the Golgi vesicles.
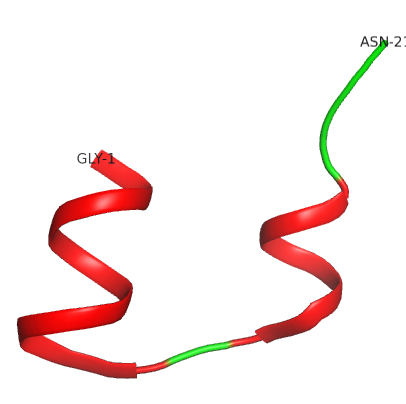
The mature insulin protein consists of two polypeptide chains (A- and B-chains). The A-chain is composed of 21 amino acids, the B-chain of 30 amino acids.
Primary
The mature insulin protein consists of two polypeptide chains (A- and B-chains). The A-chain is composed of 21 amino acids, the B-chain of 30 amino acids (find sequences below).
Task:
Compare the A-chains (and the B-chains) of human and pig insulins in the following activity by aligning their amino acid sequences. Are there any differences in the amino acid sequences?
- Copy the two amino acid sequences of human insulin and pig insulin which you find below. Select and copy the whole block of sequences starting with >sp for the A-chains (step 1) and then for the B-chains (step 2).
- Paste the sequences into the MUSCLE search box (shortcut Ctrl. + V).
- In the “STEP 2″ section ensure that “Output Format” is set at “ClustalW”.
- Click on the large “Submit” button and your alignment will be processed.
- You will see the results after a few seconds. Have a look at the alignment and answer the questions in the quiz.
Note: In order to submit the second set of sequences you may need to refresh your page and the MUSCLE search box will be empty again.
Step 1 – Compare the A-chains:
“>>sp|P01308|90-110, Insulin A-chain, Human
GIVEQCCTSICSLYQLENYCN
>sp|P01315|88-108, Insulin A-chain, Pig
GIVEQCCTSICSLYQLENYCN
Step2 – Compare the B-chains:
>sp|P01308|25-54, Insulin B-chain, Human
FVNQHLCGSHLVEALYLVCGERGFFYTPKT
>sp|P01315|25-54, Insulin B-chain, Pig
FVNQHLCGSHLVEALYLVCGERGFFYTPKA
Secondary
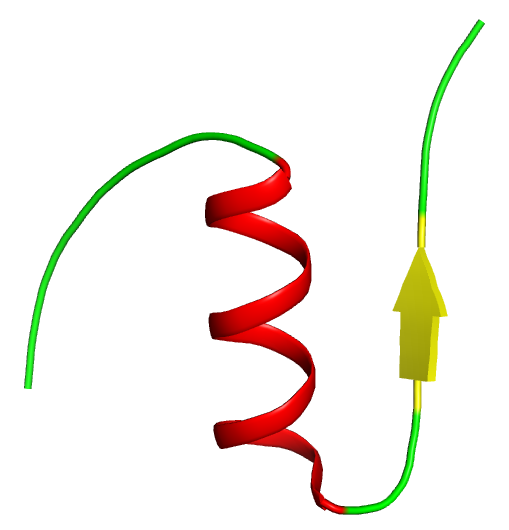
The B-chain has a major a-helix section and a beta-sheet region. It folds sharply around the A-chain (see tertiary structure).
Tertiary
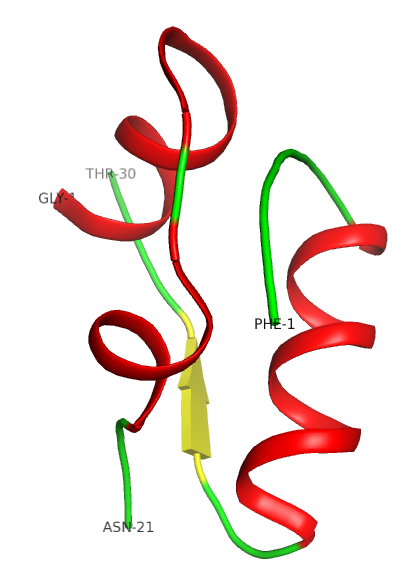
The tertiary structure is stabilized by disulphide bridges (see insulin worksheet). The external part of the protein is polar, while internally it is mostly hydrophobic.
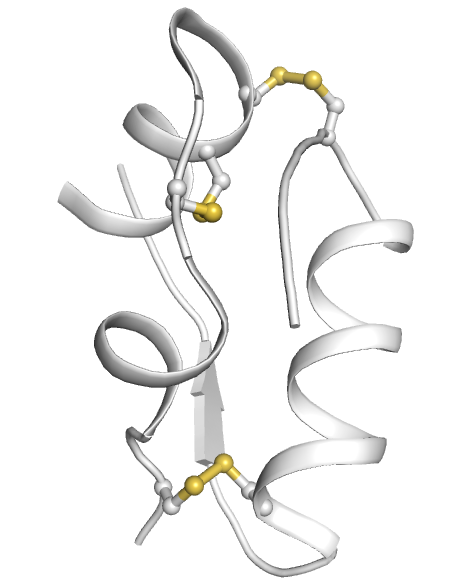
Insulin is formed by two polypeptide chains (A- and B-chains), held together by two disulphide bridges; a third disulphide bridge is situated within the A-chain.
Quaternary
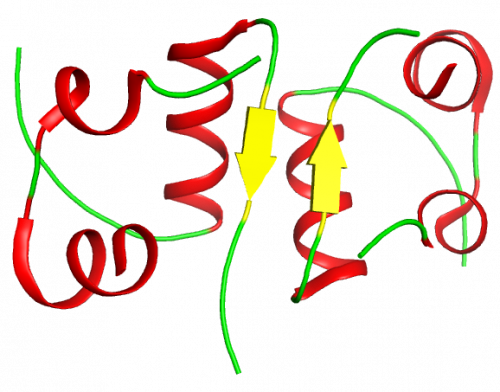
As far as the quaternary structure is concerned insulin tends to form dimers in solution, owing to the formation of hydrogen bonds between the C-terminal ends of the B-chain.
Worksheet
Download and print worksheet:
Tasks
- Use your stereo glasses to explore the protein structure in 3D (upper left).
- Color Insulin A-chain according to the color scheme below.
- Color Insulin B-chain according to the color scheme below.
- Color the following structures in the Insulin protein structure on the right:
- alpha helices
- beta sheets
- disulfide bonds
Coloring scheme:
- alpha helix – red
- beta sheet – yellow
- unstructured parts of the protein – green
- Disulfide bonds – orange
- N-terminus of protein – blue
- C-terminus of protein – purple
Quiz
Take the revision quiz and test your knowledge on this amazing molecule.
Optional material
Watch the EMBL Insight Lecture 2013 “From Code to Function – Observing Protein Synthesis Through the Electron Microscope”
Christiane Schaffitzel and her team study the function and three-dimensional structure of ribosomes using a combination of molecular biology, biochemistry and cryo-electron microscopy. In her talk, Christiane gives an overview of the steps involved in protein synthesis and the role ribosomes play in the process. She then illustrates how her research group uses cryo-electron microscopy to study the structures of these fascinating molecules. Christiane’s central research questions are: what we can learn from the make up of these fascinating molecules and how are synthesised proteins targeted to their destinations?
Topic area: Structural & Computational biology, Cell biology
Age group: 16-19
Author: ELLS Team
Share:
 Deutsch
Deutsch Français
Français Italiano
Italiano