Shape-shifting shell
As a retrovirus matures, the two parts of its shell protein (red and blue or yellow and blue) dramatically rearrange themselves, twisting and moving away from each other. (Credit: EMBL/T.Bharat)
Scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have for the first time uncovered the detailed structure of the shell that surrounds the genetic material of retroviruses, such as HIV, at a crucial and potentially vulnerable stage in their life cycle: when they are still being formed. The study, published online today in Nature, provides information on a part of the virus that may be a potential future drug target.
Retroviruses essentially consist of genetic material encased in a protein shell, which is in turn surrounded by a membrane. After entering a target cell – in the case of HIV, one of the cells in our immune system – the virus replicates, producing more copies of itself, each of which has to be assembled from a medley of viral and cellular components into an immature virus.
“All the necessary components are brought together within the host cell to form the immature virus, which then has to mature into a particle that’s able to infect other cells” says John Briggs, who led the research at EMBL. “We found that when it does, the changes to the virus’ shell are more dramatic than expected.”
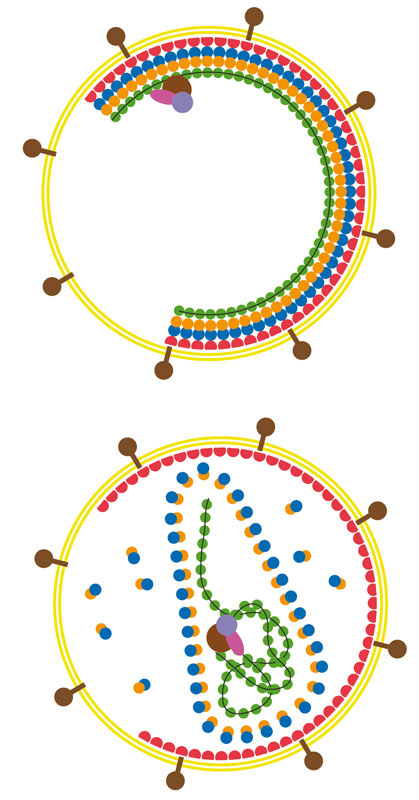
Both the mature and immature virus shells are honeycomb-like lattices of hexagon-shaped units. Using a combination of electron microscopy and computer-based methods, Briggs and colleagues investigated which parts of the key proteins stick together to build the honeycomb of the immature shell. These turned out to be very different from the parts that build the mature shell. This knowledge will help scientists to unravel how the immature virus is assembled in the cell and how the shell proteins rearrange themselves to go from one form to the other.
Findings such as these may one day prove valuable to those wanting to design new types of anti-retroviral therapies. Many anti-retroviral drugs already block the enzyme that would normally separate components of the immature shell to allow it to mature. But there are currently no approved drugs that act on that shell itself and prevent the enzyme from locking on.
Although the virus shells imaged in this study were derived from the Mason-Pfizer monkey virus and made artificially in the laboratory, they closely resemble those of both this virus and HIV – which are very similar – in their natural forms.
“We still need a lot more detailed information before drug design can really be contemplated,” Briggs concludes, “but finally being able to compare mature and immature structures is a step forwards.”
The work was done in collaboration with the group of Tomas Ruml at the Institute of Chemical Technology in Prague, Czech Republic.
For more on the Briggs group’s previous work on the structure of immature HIV.
Source article
Bharat, T.A.M., Davey, N.E., Ulbrich, P., Riches, J.D., Marco, A., Rumlova, M., Sachse, C., Ruml, T. & Briggs, J.A.G. Structure of the immature retroviral capsid at 8Å resolution by cryo-electron microscopy. Nature, advance online publication 3 June 2012.
DOI: 10.1038/nature11169.
Article abstract
The assembly of retroviruses such as HIV-1 is driven by oligomerization of their major structural protein, Gag. Gag is a multi-domain polyprotein including three conserved folded domains MA (matrix), CA (capsid) and NC (nucleocapsid) 1. Assembly of an infectious virion proceeds in two stages. In the first stage, Gag oligomerization into a hexameric protein lattice leads to formation of an incomplete, roughly spherical protein shell that buds through the plasma membrane of the infected cell to release an enveloped immature virus particle. In the second stage, cleavage of Gag by the viral protease leads to rearrangement of the particle interior, converting the non-infectious immature virus particle into a mature infectious virion 2. The immature Gag shell acts as the pivotal intermediate in assembly and is a potential target for anti-retroviral drugs both to inhibit virus assembly and to disrupt virus maturation 3. Detailed structural information on the immature Gag shell has however, not been available. For this reason, it is unclear what protein conformations and interfaces mediate the interactions between domains and therefore the assembly of retrovirus particles, and what structural transitions are associated with retrovirus maturation. Here we have solved the structure of the immature retroviral Gag shell from Mason-Pfizer monkey virus using a hybrid cryo- electron microscopy and tomography approach. The 8Å resolution structure permits a pseudo-atomic model of CA in the immature retrovirus to be derived, which defines the protein interfaces mediating retrovirus assembly. We find that transition of an immature retrovirus into its mature infectious form involves dramatic rotations and translations of CA domains, that the roles of the N- terminal and C-terminal domains of CA in assembling the immature and mature hexameric lattices are exchanged, and that the CA interactions that stabilize the immature and mature viruses are almost completely distinct.