Read the latest Issue
Lighting up development
A PhD student in the De Renzis group describes how optogenetics helped her to illuminate the path that tissues follow to get into shape
One of the most challenging experiences of my life was getting started with my PhD at EMBL, to study how organisms develop their shape, a process known as morphogenesis. Trained as a microbiologist, I was used to working with tiny single cells that displayed a rather simple behaviour. In the De Renzis group, I had to deal with fruit fly embryos made of thousands of cells that constantly exchange information and influence each other.
Understanding what is going on when an organism such as a fruit fly develops from egg to larva is a daunting task. Much of what we know about the biological processes that drive development comes from turning specific genes on and off and then watching for the effect. However, the commonly used techniques have a limitation: one has to wait some time (several hours in the best case) before looking at the consequences of such perturbations and drawing conclusions. As my supervisor Stefano De Renzis puts it, “it’s like arriving at the scene of a car crash: you know something went wrong at some point, but you were not there when it happened, so you cannot tell what exactly caused the accident”.
When I joined Stefano’s lab, my dream was to have a remote control to perturb the activity of single cells, and watch the effects immediately. That way, I thought, it would be much easier to understand when and where specific cell behaviours were needed during complex developmental processes. I thought that a feasible way to create such a remote control would be to use light. Back in 1999, Francis Crick had already voiced the possibility of controlling neuron activity with light. Just a couple of years later, neuroscientists managed to use light to activate brain cells, and they named the newborn technique ‘optogenetics’. However, when I started my PhD in 2011, no one had ever used this technique to interrogate how cells behave and interact with each other in a whole organism during development.
I have always had a strong interest in biotechnology, and introducing optogenetics as a tool to study embryonic development seemed like the ideal PhD project for me. As an embryo develops, most of the changes in its shape are driven by cells contracting, so I decided to prevent them from doing so, by using light to reduce their ‘muscle fibres’. Work from our and other groups had shown that a particular fatty molecule in the cell membrane – a lipid called PI(4,5)P2 – serves as an anchor for the fibres that pull on the membrane when the cell contracts, and is essential for many developmental processes. Inspired by these findings, I set up an approach to use light to remove PI(4,5)P2 from the cell membrane, as this would prevent the fibres from latching on, meaning the fruit fly embryo’s cells would be unable to contract. One of the most exciting moments of my PhD was the day I went to the microscope and confirmed that I could stop individual cells from contracting with just a few millisecond pulses of light: my light-based remote control was working!
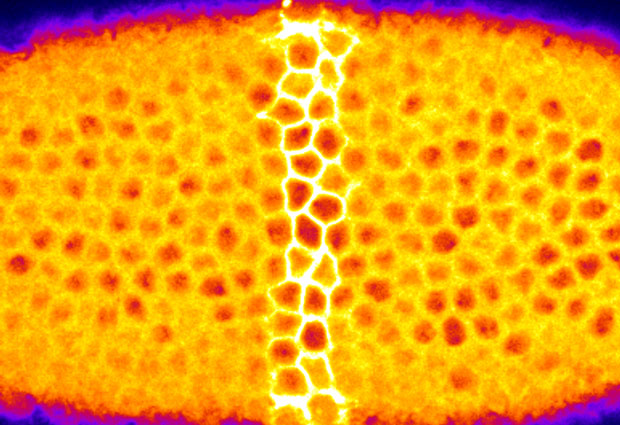
In the meantime, working close to morphogenesis enthusiasts in both Stefano’s group and the neighbouring Leptin lab, I got interested in how tissues bend to form folds or tubes. At around 4 hours old, for instance, cells on the underside of the fruit fly embryo start moving inwards, folding into the embryo where they will eventually give rise to muscles. By using my brand new optogenetic remote control to block cell’s ability to contract, I found that there is a group of cells that is absolutely required for the tissue to fold inwards. What was even more fascinating was that the geometry in which these cells are organized within the tissue sets up the way they change their shape. It’s like when you bake a cake: it will take the shape of the tin you poured the mixture into. In the same way, the geometry of the tissue imposes some sort of constraint on the way individual cells behave.
When these results came to light, I needed to transform a lot of visual information into meaningful numbers, so I teamed up with Joseph Barry, then a postdoc in Wolfgang Huber’s group. Joe did an amazing job of developing algorithms to quantify cell features and discussing the huge amount of data I had produced: “For me the best parts were sitting together mulling over images, trying to figure out what was going on,” he now recalls.
The paper in which we describe this new optogenetic method to control cells’ ability to contract, and its application to study fruit fly morphogenesis is just published today in Developmental Cell, but collaborations with labs worldwide have already started. “Other scientists asked for help and reagents to modulate stem cell activity and to control neural tube formation in vertebrates,” explains Stefano, “In fact, cell contractility is a highly conserved cell behaviour that drives many morphogenetic processes, so I believe that this optogenetic approach will be applicable to other organisms to address questions about cell-cell interaction and tissue mechanics.”
My adventure as a PhD student at EMBL is almost finished, but Stefano has long-term plans to use light to quiz other key events that shape the embryo: “What we are doing now is engineering this optogenetic system to be able to control signalling between individual cells during morphogenesis and get an insight into cell communication. We want to know what kind of information cells exchange, when they exchange such information and how loud they ‘speak’, for instance.”
Keep an eye out: optogenetics will make you see development in a new light!