Fiona M. Watt
EMBO Director
fiona.watt [at] embo.org
ORCID: 0000-0001-9151-5154
EditRegulation of stem cell fate

EMBO Director
fiona.watt [at] embo.org
ORCID: 0000-0001-9151-5154
EditThe focus of our research is mammalian skin, which we study using human cells in culture (Hiratsuka et al., 2020) and genetically modified mice (Sipilä et al., 2022). In early studies, we found markers to isolate epidermal stem cells and elucidated several of the signalling pathways that regulate stem cell behaviour, two of which – integrin and Notch signalling – are a focus of our current research. We have uncovered remarkable plasticity of epidermal stem and differentiated cells and discovered ways in which epidermal-dermal communication controls skin homeostasis. We make extensive use of single-cell analysis (Reynolds et al., 2021) and are defining how biophysical cues elicit transcriptional responses in epidermal stem cells. Having demonstrated the existence of different skin fibroblast lineages in mice, we are analysing different subpopulations of human skin fibroblasts, with the goal of developing new strategies to treat skin scarring.
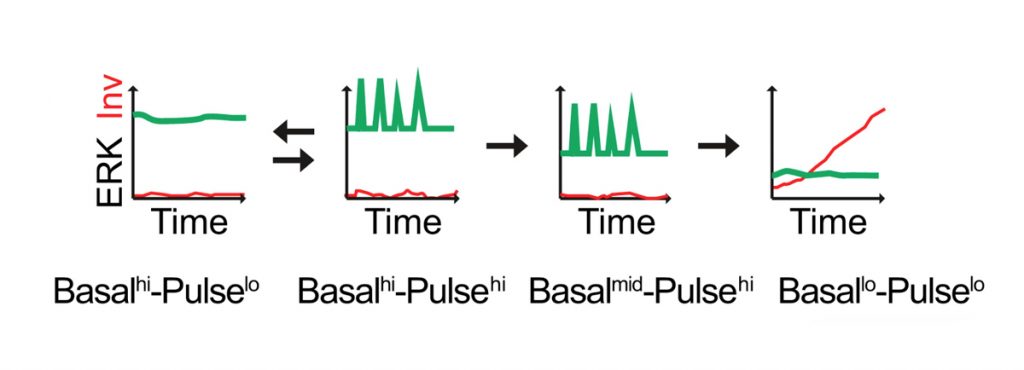
Our current goal is to explore the interplay between ERK MAPK and Notch signalling dynamics in regulating epidermal stem cell fate in real time at single-cell resolution. Integrin-mediated signalling via ERK MAPK plays a central role in stem cell maintenance and exit from the stem cell compartment is executed by an unstable switch comprising an interacting network of protein phosphatases (PMID: 29043977). We have explored the dynamics of the onset of differentiation by transducing keratinocytes with a FRET sensor of ERK activity and an mCherry fluorescent reporter of expression of the differentiation marker, involucrin, thereby enabling live cell imaging (Hiratsuka et al., 2020; Figure 1).
Another mechanism that regulates epidermal stem cell maintenance is expression of the Notch ligand Dll1. Stem cells express higher levels of Dll1 than their neighbours. Dll1 protects stem cells from differentiating, promotes stem cell clustering, and provides a signal to neighbouring cells to differentiate (PMID: 31346203). In contrast, Jag1 promotes epidermal differentiation. Just as ERK signalling involves pulses of activity, the same is true for Notch signalling. By transducing human keratinocytes with Notch activity reporters, we will examine whether the different outcomes of Dll1 and Jag1 stimulation reflect differences in signal dynamics.
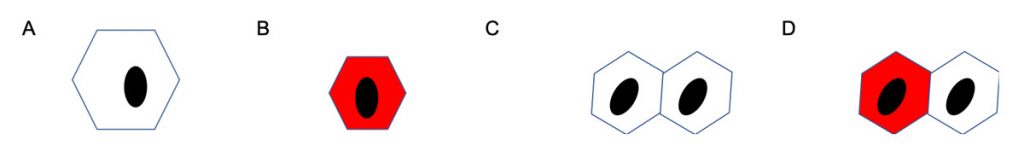
To carry out our investigations, we will use micro-patterning technology (Vickers et al., 2021) to create hexagonal ECM substrates that control cell spreading and cell-cell contact (Figure 2). When cells are plated on small bowtie-shaped micropatterned islands comprising two adjoining hexagons, differentiation is significantly reduced, and only one cell in each pair expresses involucrin. The strongest candidate mediator of this asymmetric effect is the Notch ligand Delta-like 1.
By combining the new bowtie micropatterns with available sensors of ERK and Notch signalling, we can examine the interplay between cell-cell and cell-ECM adhesion in regulating differentiation in real time. This will not only define the earliest events in the onset of differentiation but also potential interactions between two of the key signalling pathways that regulate epidermal homeostasis.