Hannah Stuart
Group Leader (incoming)
ORCID: 0000-0001-9563-9760
EditSelf-organisation in the central nervous system

Group Leader (incoming)
ORCID: 0000-0001-9563-9760
EditAnimals are built from many different cell types, exquisitely organised in space and time to make functioning organs that work together. Starting from a single zygote, how do cells diversify and coordinate with each other to create a complex embryo? Cells make decisions based on their internal state (cell-autonomous) and external cues (non-autonomous). Order can emerge in the absence of a pre-existing “blueprint” as a result of local interactions between cells (self-organisation). By bridging scales from molecular governance of stem cell decision-making to emergent tissue properties, we aim to understand fundamental biology and uncover minimal design rules to build and re-build tissues.
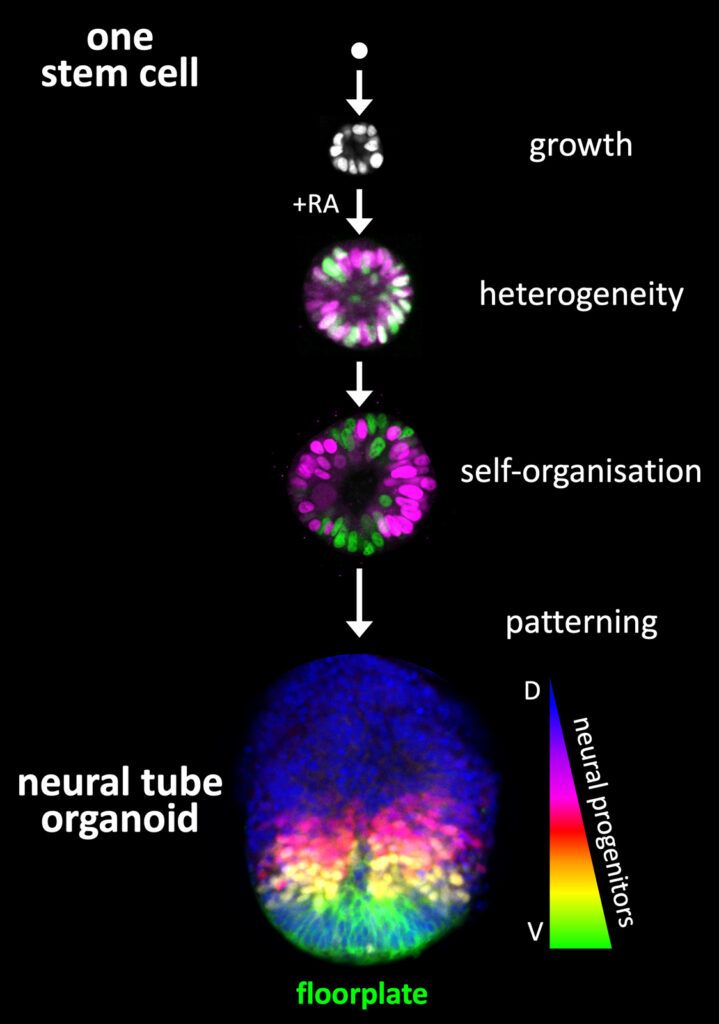
In mammals, the mechanisms by which self-organisation contributes to development are challenging to study, due to the embryo’s complexity and in utero location. In vitro stem cell models (organoids, embryoids) can spontaneously break symmetry and create patterns of cellular differentiation, allowing deconstruction and reconstruction of developmental mechanisms in a controlled setting. We use neural tube organoids (NTOs) (Figure 1) to study principles of multicellular self-organisation and as a basis for tissue engineering. The neural tube is the embryonic precursor of the brain and spinal cord. Starting from a single cell, mouse NTOs form 3D tissues that recapitulate the highly conserved pattern of neural progenitors and neurons critical for central nervous system form and function (Figure 1).
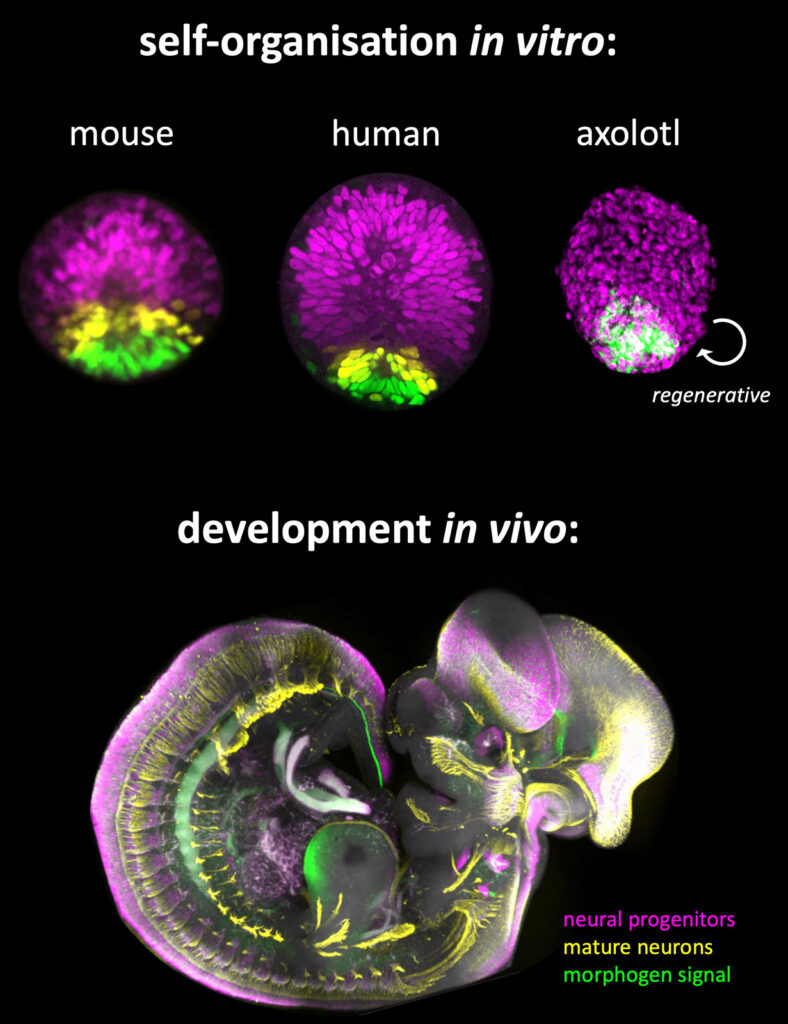
Unlike mammals, the axolotl salamander can regenerate patterned spinal cord tissue after injury. We created axolotl spinal cord organoids that can repeatedly self-organise (Figure 2), in contrast to mammalian NTOs that have only one window of opportunity during their developmental trajectory. We have also established human NTOs, for the dual role of inter-species mechanistic comparison and advanced human tissue engineering (Figure 2).
Our goals are to understand how the self-organising properties of stem cells contribute to the robustness of vertebrate development and regeneration, and to deconstruct the interaction between self-organisation and context. We employ an interdisciplinary approach, integrating advanced stem cell manipulation, mammalian and amphibian neural organoids, computational modelling, embryology, and bioengineering.
Our projects include: