A new paper on the U5 snRNP assembly
U5 snRNP is one of the key building blocks of the spliceosome, but how it is assembled and recycled remains unknown.
In our new paper published in NSMB, we provide new mechanistic insights into this process.
https://www.nature.com/articles/s41594-024-01250-5
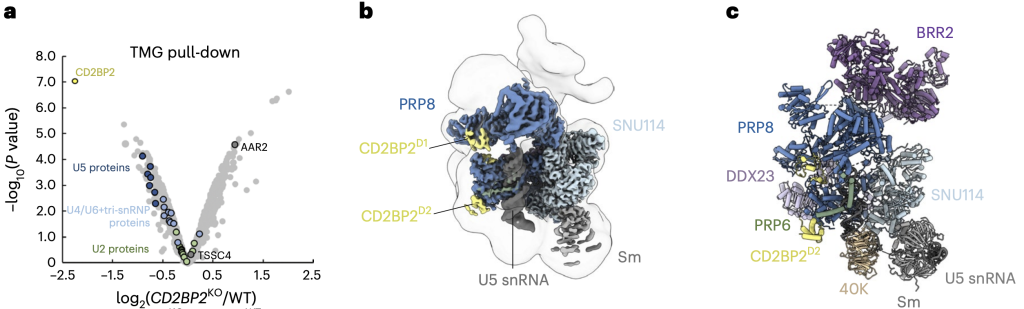
We investigated the role of the assembly factor CD2BP2 in the U5 snRNP and tri-snRNP biogenesis. To do so, we created a CD2BP2-deficient human cell line using CRISPR/Cas9 gene editing and analysed the composition of snRNPs purified from this cell line by quantitative proteomics. We found subtle defects in the U5 snRNPs and U4/U6.U5 tri-snRNPs formation due to a roadblock in their assembly pathway and accumulation of the AAR2-containing precursor complex.
We purified U5 snRNP bound to CD2BP2 and determined its structure by cryoEM. The structure shows how CD2BP2 stabilises the conformation of two mobile domains of PRP8 and prevents the pre-mature binding of the U4/U6.U5 tri-snRNP-associated factor DIM1. Our findings emphasise the role of the assembly factors in orchestrating the sequential utilisation of overlapping protein-protein interfaces during tri-snRNP maturation.
Great team effort from multiple lab members, including Sarah Schneider, Irina Brandina, and Daniel Peter.