Talya Dayton
Group Leader
ORCID: 0000-0002-7994-7963
EditOrganoid models of neuroendocrine development and cancer

Group Leader
ORCID: 0000-0002-7994-7963
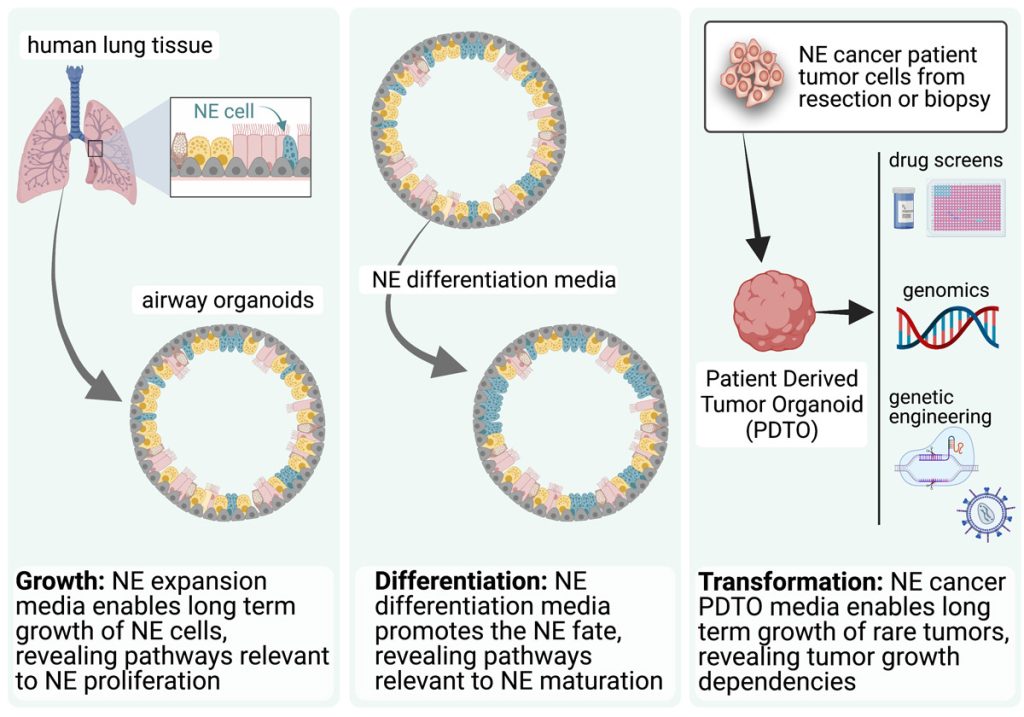
EditOur group uses organoids to understand: (1) differentiation and plasticity of NE cells, and (2) the biology of NE cancers (Figure 1).
Differentiation and plasticity of NE cells
Pulmonary NE cells account for only 0.5% of the lung epithelium. They secrete hormones and neuropeptides that influence lung physiology, and multiple studies show increased pulmonary NE cell numbers in human diseases, suggesting an important function. These cells are also a cell of origin for pulmonary NE neoplasms, a tumour type that constitutes 20-25% of invasive primary lung cancers.
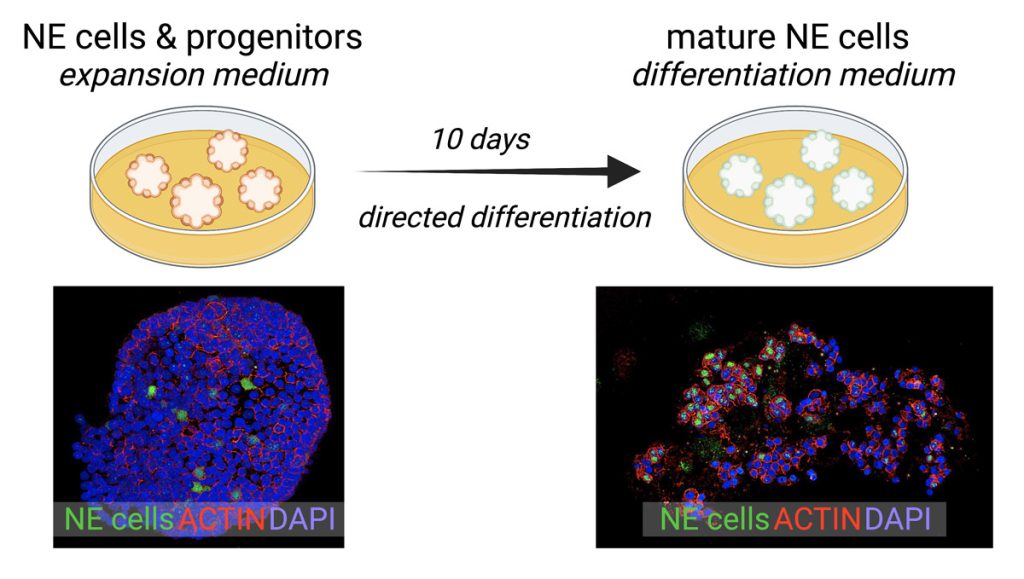
We have pioneered a tractable and robust model system for the study and manipulation of human pulmonary NE cells and their progenitors in multicellular organoids that also contain all of the major airway cell types (Figure 2).
Biology of NE cancers
NE neoplasms comprise NE tumours (NETs) and NE carcinomas (NECs). While NETs generally proliferate and progress slowly, NECs are associated with a very poor prognosis. Irrespective of subtype, the treatment options for patients with unresectable, relapsed, or metastasized NE neoplasms are limited. We have developed novel patient-derived tumour organoids (PDTOs) of pulmonary NETs and of large cell NEC (LCNEC) that conserve and maintain the intratumoral heterogeneity and major gene expression patterns observed in matched tumour tissue. Using these PDTO models we:
Broadly, we aim to tackle several outstanding questions:
(1) Does heterogeneity in the molecular states that lie along the differentiation trajectories for NE cells influence genomic and phenotypic intratumour and inter-tumour heterogeneity in NE cancers?
(2) How do NE cells respond to acute and chronic injury and how do these responses change when they acquire cancer-associated mutations?
(3) How do cancer-associated mutations influence cell state and differentiation in NE cells and NE cell progenitors?
More specific goals include:
Seminario Frontiers in Genomics 2025 – Frontiers in Genomics