Hiroki Asari
Group Leader (Incoming/Outgoing)
hiroki.asari [at] embl.it
ORCID: 0000-0003-3396-1935
EditVisual systems neuroscience

Group Leader (Incoming/Outgoing)
hiroki.asari [at] embl.it
ORCID: 0000-0003-3396-1935
EditOur research has been centered around the following three questions:
1. How does computation arise from interactions of neurons?
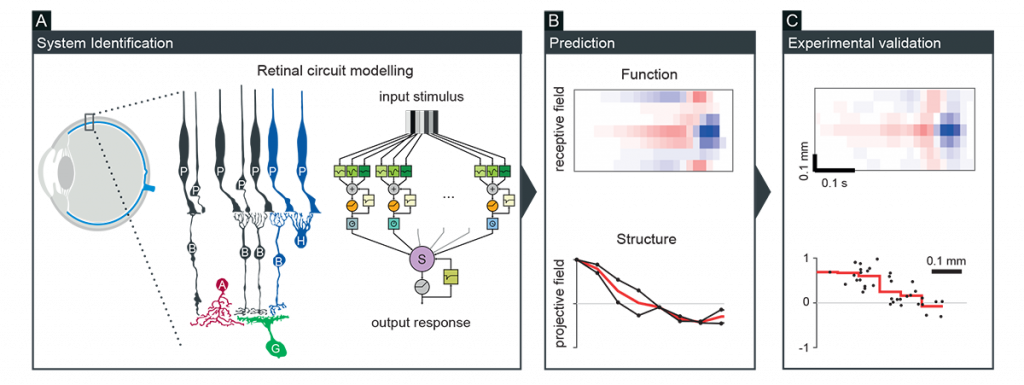
Neurons form various interconnected circuits to perform diverse computations. We have been taking a theory-driven approach to better understand the relationship between structural connectivity and neuronal dynamics. For example, we developed a biologically faithful but computationally tractable model of the retina, from which predictions were derived on the circuit structure and function, and subsequently tested for experimental validation (Figure 1; Real et al., Curr Biol 2017; Vlasiuk and Asari, PLoS One 2021). We also applied such a modelling approach to better understand the hypothalamic circuit function underlying mouse innate behavior (Rahy et al., Front Comput Neurosci 2022). These results showcase a power of the circuit modelling approach to gain deeper insights into neural information processing.
2. What does the eye tell the brain in vivo?
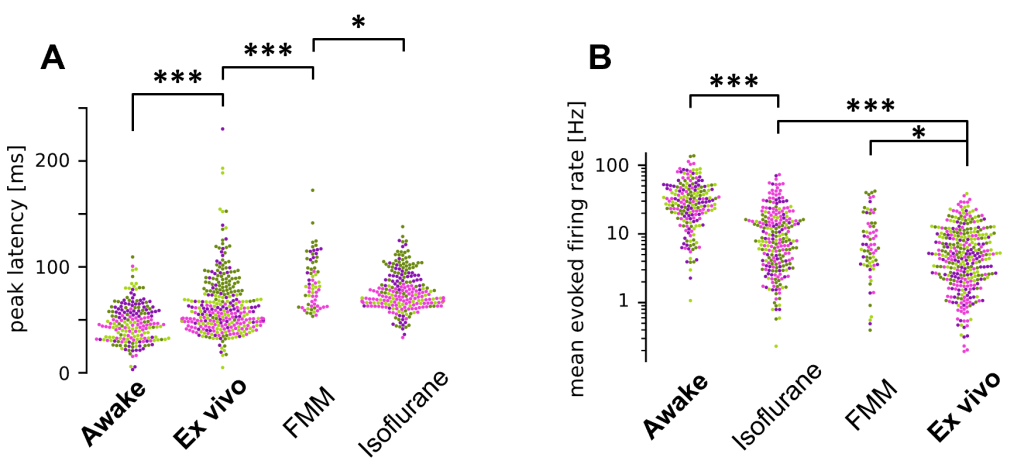
Retinal signaling has been well studied ex vivo, with an assumption that findings from these studies should be naturally translated to how the retina operates in vivo. Using single-unit electrophysiological recordings from the mouse optic tract, we directly monitored retinal output in vivo, and found that the retinal response properties are substantially different in awake mice from those in anesthetized or ex vivo conditions (Figure 2: Boissonnet et al., eLife 2023). This challenges the common assumption in the field, and idicates a need for further studying retinal function in vivo.
To better understand how retinal signals are transmitted to the downstream brain areas, we also functionally mapped the spatial organization of the input axons and target neurons in the mouse retinocollicular pathway at single-cell resolution using in vivo two-photon calclium imaging. We found a near-perfect retinotopic tiling of the retinal ganglion cell axon terminals, showing topographically more precise organization than local neurons in the superior colliculus (Molotkov, Ferrarese, et al., Nat Commun 2023). This highlights the precision of brain wiring, and raises a possibility that the nervous systems are wired more precisely than previously thought to exploit topographic information for their function.
3. How is visual processing altered in disease conditions?
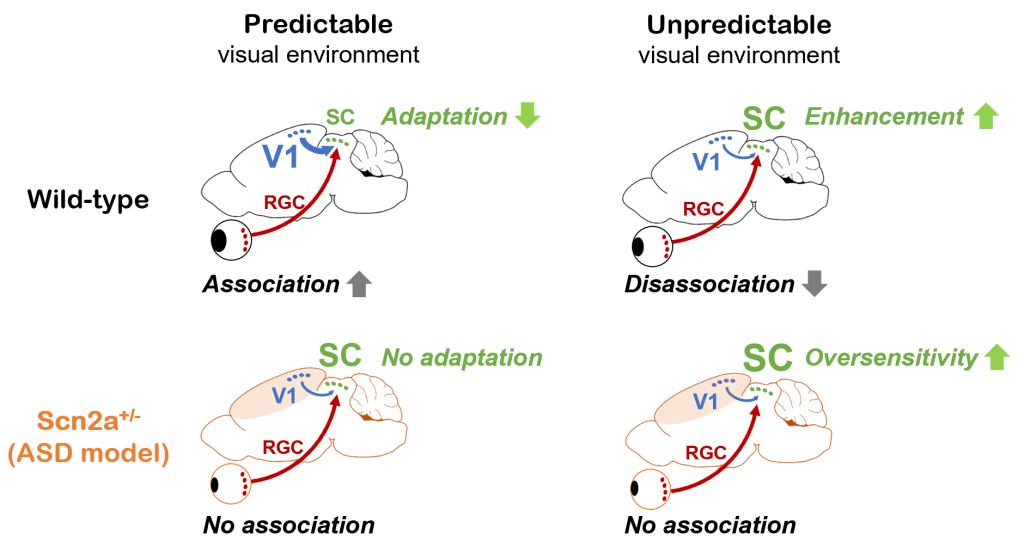
While an animal’s behavior can modulate visual processing, the visual system in turn can affect behavior by predicting future events based on current sensory information and past experiences. Anomaly in such inference process is thought to underlie various symptoms in psychiatric conditions, and we indeed found sensory expection mismatch in a mouse model of autism at both neurophysiological and behavioral levels (Ferrarese and Asari, bioRxiv 2023). Specifically, mice carrying a genetic mutation that causes autism in humans cannot fully exploit past experiences to update visual sensory responses; and this phenotype derives from a deficit in corticotectral feedback in the visual system (Figure 3). In a critical cross-species follow-up, we also found that the performance of the autism model mouse closely paralleled that of the corresponding human subjects when analyzed via a Bayesian statistics methodology. While we have thus far focused on a specific brain region of a specific mutant mouse involved in a specific computation, we expect that our findings may extend as a more generic computational feature of psychiatric conditions that involves complex, recursive interactions of sensory and associative systems in the Bayesian inference framework.
Based on the results we obtained in the past years, we will extend our current research program to further characterize early visual processing in awake mice. Specifically, we will focus on the following two questions:
Everything that the brain sees depends on the retina. To better understand how our visual percept arises under different behavioral contexts, it is indispensable to characterize retinal signals as an integrated part of the entire nervous system, but not in isolation. The retina shares many common features with the rest of the brain, such as diverse cell types and neuromodulators, laminar organization, and spatial proximity of neurons that convey similar information. Thus, studying retinal function in vivo will provide key insights into not only early visual processing, but also how networks and physiological properties of neurons are combined to perform complex and adaptive computations.
We will also continue to analyze disease model mice in our experimental paradigm. We envision that systems neuroscience approach to sensory dysfunction is a promising future direction to find a link between genetic, behavioral, and neurophysiological phenotypes.