Best Poster Awards – In Situ Structural Biology Workshop
The EMBO Workshop: In Situ Structural Biology: From Cryo-EM to Integrative Modelling was our final virtual conference of 2020, but there was no trace of Zoom fatigue amongst the 466 participants who joined us from 6 – 8 December!
80 international researchers presented their posters during the two posters sessions on the following topics:
- Biophysical analysis in cells
- COVID-19
- Imaging across scales
- Integrative modelling
- Molecular sociology
- Structural analysis in situ
- Structural biology
Each of the participants had the chance to vote for their favourite poster, resulting in two posters winning the Best Poster Award kindly sponsored by EMBO Press. Here are the winners:
New insights on the catalytic mechanism of arsenite oxidase

Authors: Filipa Engrola, Márcia Correia, Teresa Santos-Silva, Maria Romao, (UCIBIO@FCT-NOVA, Portugal)
Arsenic (As) and antimony (Sb) are two metalloids that, due to anthropogenic and natural causes, pose an environmental threat, considered as priority pollutants by the World Health Organisation and the United States Environmental Protection Agency. Although the safety guards recommend a maximum of 10 μg/L of As and Sb in drinking water, these values are exceeded in many regions worldwide, with no remediation approach that is simultaneously effective, clean and economically sustainable [1,2]. The ancient bioenergetic enzyme arsenite oxidase (Aio), from microorganisms Rhizobium sp. NT-26 (NT-26 Aio) and Alcaligenes faecalis (A.f. Aio), is currently being studied for its use as a biosensor and in bioremediation processes. Both Aio enzymes contain a large subunit (AioA) that harbours a molybdenum centre and a [3Fe-4S] cluster, and a small subunit (AioB) that possess a Rieske [2Fe-2S] cluster and have demonstrated to oxidise AsIII, as well as SbIII, into the easier to remove and less toxic forms of AsV and SbV, respectively [3,4]. Aiming to elucidate the catalysis mechanism of the enzymes, a combination of expression and purification of the proteins, crystallisation, structural analysis, enzyme kinetics and affinity tests were conducted. X-ray structures of the ligand-free form of the enzyme had been previously determined (PDB: 4AAY, 5NQD and 1G8K [3,5,6]). In our work, Aio crystals in complex with two different forms of the substrate analogue – Sb oxyanions, with a reaction kinetic 6500 times slower than AsIII [6] – diffracted up to ca 1.8 Å resolution. The structures show the reaction intermediates bound at the active site, with a μ-oxo bridge binding Sb to the Mo atom. Analysis of bond lengths and geometry of the ligands at the Mo active site allowed us to revisit the catalytic mechanism of As oxidation [7], contributing to the understanding and future biotechnological application of this family of enzymes in water treatment.
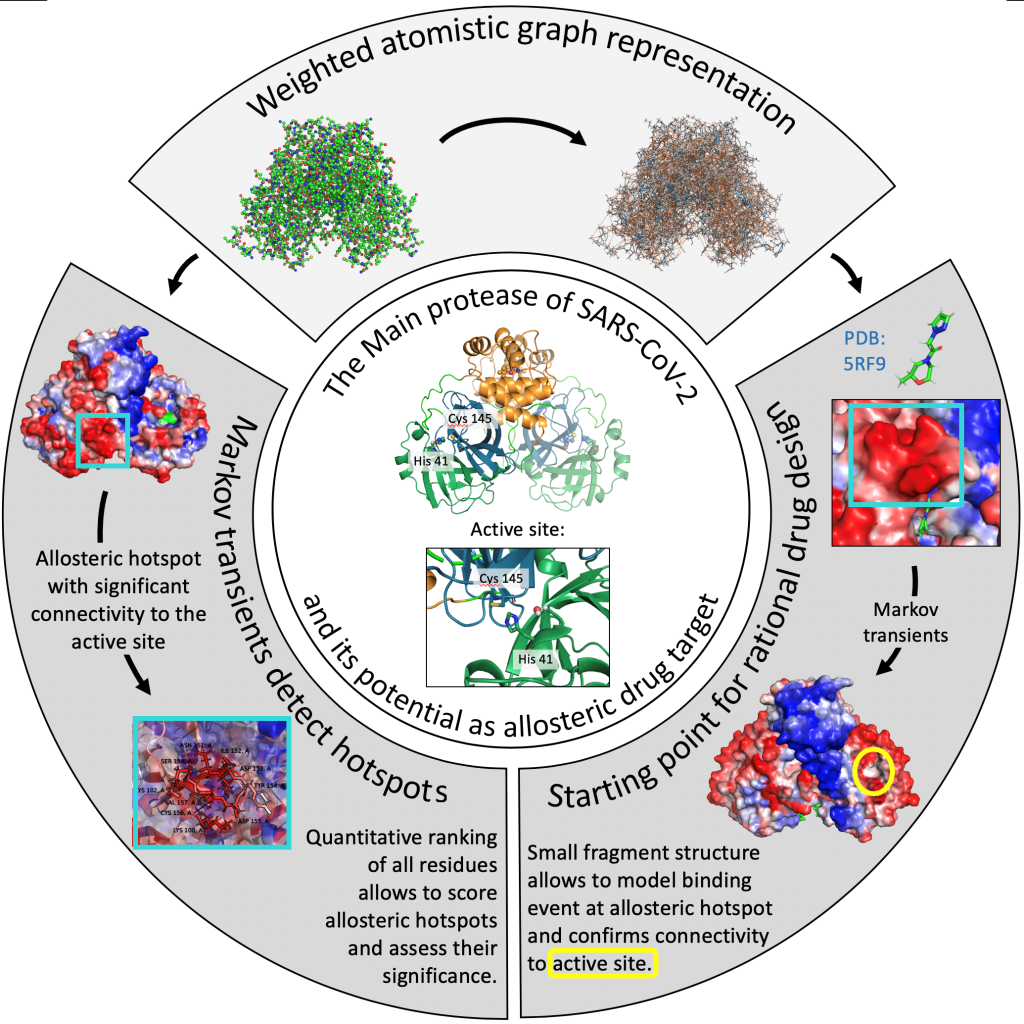
Allosteric hotspot in the main protease of SARS-CoV-2

Authors: Léonie Strömich, Sophia N Yaliraki, (Imperial College London, UK)
Since the beginning of 2020 we have seen the coronavirus SARS-CoV-2 causing a global pandemic with almost 34 million cases and over 1 million deaths worldwide [as of 01.10.2020] [1.] As a result, we have seen a surge in research efforts to develop effective treatments for the underlying disease, COVID-19. One approach is to target the main protease (Mpro) of SARS-CoV-2 as it is essential for virus replication in an early step of the viral life cycle [2.] Most efforts are centred on inhibiting the orthosteric binding site of the enzyme. However, considering allosteric sites on the protein allows for more selective drug design and widens the chemical search space. Here, we report an allosteric hotspot in the SARS-CoV-2 Mpro dimer by using novel atomistic graph theoretical methods: Markov transient analyses follow the propagation of a random walker on a graph and have been shown to successfully identify allosteric communication in catalytic proteins [3.] We further score the so identified allosteric hotspots against random sites in similar distances and thus identify a statistically significant putative allosteric site in the SARS-CoV-2 Mpro. We then simulate a binding event at this hotspot region using data from a recent XChem fragment screen by the Diamond Light Source [4.] which provides a starting point for rational drug design. This study uses highly efficient network theoretical models to shed light on allosteric communication and uncovers putative allosteric sites in the SARS-CoV-2 main protease. This provides a valuable contribution to the ongoing efforts to find a cure against COVID-19 by broadening the horizon for drug discovery efforts.
References:
[1.] Official World Health Organization COVID-19
dashboard: https://covid19.who.int (Accessed: 01.10.2020).
[2.] Hilgenfeld, R. (2014). FEBS Journal, 281(18), 4085-4096.
[3.] Amor, B., Yaliraki, S. N., Woscholski, R., & Barahona, M. (2014) Molecular BioSystems, 10(8), 2247-2258.
[4.] Douangamath, A., Fearon, D., Gehrtz, P., Krojer, T., Lukacik, P., Owen, C. D., … Walsh, M. A. (2020) Nature Communications, 11, 5047.
Working on your own conference poster? Then check out these 8 tips for preparing a digital poster that stands out from the crowd.