Read the latest Issue
Drugging bacteria
Commonly used diabetes drug impacts gut bacteria more than the disease itself, EMBL scientists have found.
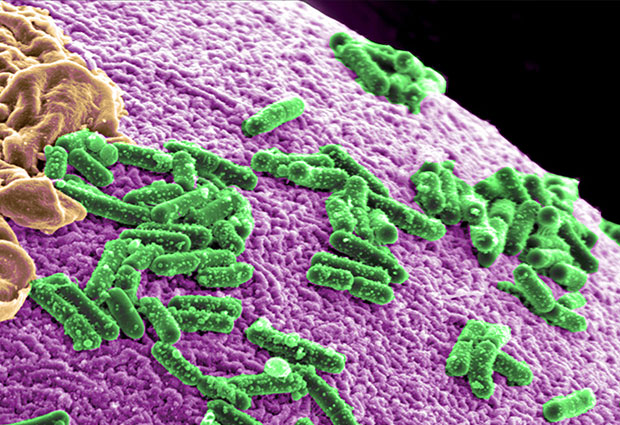
Metformin, the drug most often used to treat type 2 diabetes, has a greater effect on gut microbes than the disease itself. The finding, by the Bork group and colleagues in the MetaHIT consortium, has implications for studies searching for links between our microbiomes and disease. Published today in Nature, the study points to new approaches for understanding how metformin works, and minimising the side effects of a drug that patients take in high doses for many years.
“It’s surprising that this single drug triggers such a noticeable change in our microbes,” says Peer Bork, who led the work at EMBL. “Now think how many drugs are out there, and how many people take several drugs daily: even if only a fraction of drugs have this big an impact, they could still be dramatically shaping people’s gut.”
Metformin is a drug commonly used to treat type 2 diabetes, to help control blood sugar levels. It is typically administered in relatively high doses. And, as type 2 diabetes is a chronic disease, patients usually take the drug for years. But exactly how it acts inside patients’ bodies is not yet entirely clear.
anyone trying to minimise side effects could think about acting on the microbes
The scientists compared stool samples from over 700 people, including patients with type 2 diabetes and healthy people. The team found that, based on the communities of microbes present in a person’s stool, they couldn’t reliably tell if the person had diabetes or not – unless the person took metformin. Patients who took the drug had considerably more E.coli and fewer I. bartletti bacteria than healthy people and patients who did not take metformin.
“The differences we observed in microbial communities could explain some of metformin’s side effects, which means anyone trying to minimise those side effects could think about acting on the microbes in the gut,” says Kristoffer Forslund, from Bork’s lab.
Some day, a person taking metformin might conceivably also get a yoghurt or dietary supplement to help maintain the balance of their inner microbial community, the scientists postulate.
The findings also explain the contradictory results of previous studies trying to establish how the community of microbe species in the gut differs between healthy people and people with type 2 diabetes. The fact that different studies found different microbial communities associated with diabetes was probably because more patients took metformin in one study than in another.
we’re not alone: the medicines we swallow don’t just affect us directly
For scientists searching for bacterial ‘fingerprints’ that could be used to diagnose or treat diseases such as diabetes, the current study highlights the importance of considering whether treatments are influencing – and potentially confounding – the results.
“Our study sets a new standard for exploring the impact of various disorders on the human gut microbiota,” says Oluf Pedersen, who led the study at Novo Nordisk Foundation Center for Metabolic research at University of Copenhagen. “Ideally, future studies should be run in treatment-naïve patients to avoid confounding effects of medications on disease-specific imbalances of intestinal microbes.”
“This work really highlights the importance of the gut microbiome, not just in keeping us healthy but also in influencing how we react to treatments,” says Dusko Ehrlich, from the French Institute for Agricultural Research (INRA), in France, who led the MetaHIT consortium.
“These findings really show we’re not alone: the medicines we swallow don’t just affect us directly. They can have a profound impact on our microbial ‘inhabitants’, too,” Bork concludes.
This study stems from earlier work by – and draws data from – the MetaHIT consortium, an EU-funded project which ran from 2008 to 2012 and which aimed to establish associations between the genes of microbes in the human gut and our health and disease.