Alexander Aulehla
Head of Developmental Biology Unit and Scientific Head of LAR
ORCID: 0000-0003-3487-9239
EditTiming in embryonic development

Head of Developmental Biology Unit and Scientific Head of LAR
ORCID: 0000-0003-3487-9239
EditDuring an embryo’s journey from a single cell to a complex organism, countless patterning processes unfold with remarkable precision, spatially but also in respect to temporal sequence, or timing. This temporal aspect of embryonic development is the focus of our research. How is time measured during embryonic development and what extrinsic and intrinsic signals control this timing? How are embryonic oscillators/clocks employed during patterning? What are the dynamics of signalling pathways?
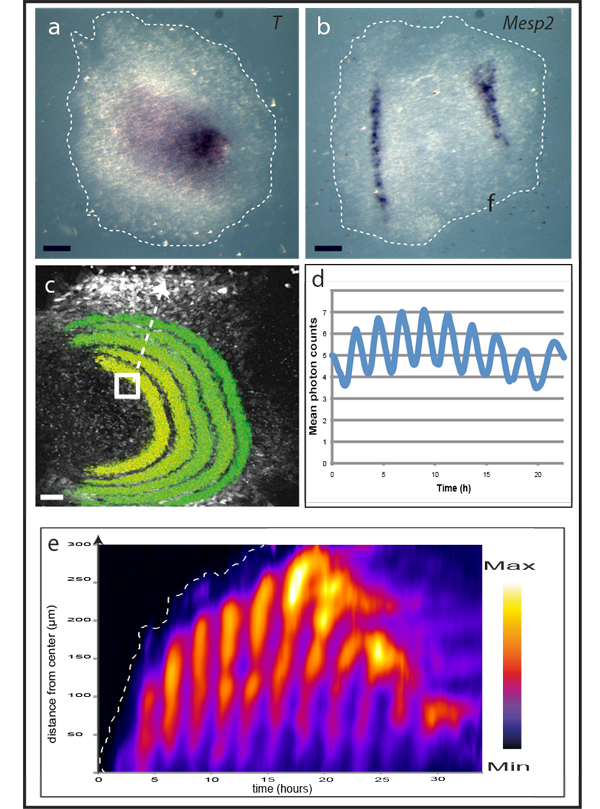
To approach these questions, novel methodologies are required (see video 1). We are generating novel real-time reporter mouse lines using knock-in technology that enables visualisation and quantification of temporal dynamics at different levels in the context of mouse embryonic development. Using in vivoimaging, we are focusing on the somite segmentation clock, an oscillatory system that is thought to control the formation of the pre-vertebrae that form periodically in a head-to-tail sequence within the paraxial mesoderm. In mouse embryos this clock, with a periodicity of around two hours, drives oscillatory activity of several signalling pathways (Wnt, Notch and Fgf signalling) in the developing mesoderm.
We recently developed an ex vivo assay that, in combination with real-time imaging reporters, has become instrumental for our approach: the assay recapitulates mesoderm patterning, including segment formation and spatio-temporally controlled oscillatory signalling activities, within the simplified context of a monolayer of primary mesoderm cells put in culture (see figure & video 2).
One fundamental property of vertebrate segment formation is its ability to maintain proportions even when overall embryo size is experimentally altered, a process termed scaling. Intriguingly, scaling behaviour can be observed in the ex vivo assay system as well. This enabled us to identify a novel scaling mechanism employing phase-shifted oscillatory activity (Lauschke et al., Nature 2013). One major interest is how temporal devices, or oscillators, mechanistically encode spatial information for patterning, particularly at an integrated, higher-order level, so as to reveal emergent properties, incorporating mathematical modelling into our approach.
This signalling pathway serves a multitude of evolutionarily conserved functions during development and has been shown to play an essential role during somite formation. Our novel real-time reporter system is designed to reflect oscillatory Wnt-signalling activity both at gene activity and at protein levels. This will enable us to determine how the striking oscillations of Wnt-signalling activity are generated in the first place and, moreover, to functionally test their role in embryonic patterning, particularly identifying the intrinsic and extrinsic factors that are responsible for controlling these oscillations within the segmentation process.