‘The human microbiome’ through the eyes of our event reporter, Aditya
Written by Aditya Malwe, IISER Bhopal, India

As a final year PhD student, I was very keen to attend the EMBO | EMBL Symposium “The human microbiome” to showcase my work as well as to establish contacts with researchers working in the field of the human microbiome across the world. This was my first time attending a conference on-site and I am really glad I got the opportunity to visit Heidelberg and attend #EESMicrobiome. I am really thankful to the speakers and presenters who presented on such diverse topics, some of which were really novel to me. The scale and importance of this conference can be highlighted by the number of participants attending on-site as well as virtually, which was very helpful for meeting fellow researchers, getting to know about different aspects of microbiome research happening around the world, and establishing contacts. Thanks to the organisers, the entire program was seamless and punctual and I would also like to thank the organisers for providing online access to recorded talks by speakers. It was a great experience and I hope to attend future editions of #EESMicrobiome.
Six symposium takeaways about human microbiome research
The relationship between the human host and microbes inhabiting various sites on and within the human body is a complex affair that has great influence on our health. Helpful microbes already in our guts protect us from pathogens, digest dietary components that are otherwise indigestible by humans, and synthesise key metabolites that have nutritive value and provide protection against diseases.
In turn, a host’s diet, lifestyle, and to some extent, genetics directly affect the human microbiota along with the environment. Changes to any of these factors can cause dysbiosis, which is a disruption in the abundance of these helpful microbes, and can even lead to disease.
In September, EMBO | EMBL held the symposium, ‘The human microbiome’, where scientists presented their latest research and techniques to study the human microbiome, explored factors that influence the human microbiome and the association of diseases with specific human microbial signatures, and discussed how the human microbiome can be used as a therapeutic target to treat disease.

Here are six symposium takeaways that highlight the depth of ongoing research on the human microbiome:
- Not just a gut feeling, but gut data to provide diet information
Changes in diet can lead to changes in the gut microbiome. Unfortunately, participants in research trials are not reliable reporters of their own diet information either because they forget or simply don’t report complete diet information. Oleksandr Maistrenko from the Royal Netherlands Institute for Sea Research presented an approach that offers at least a partial solution, made possible thanks to what the gut can tell us. He identifies specific DNA biomarkers, such as mitochondrial and plastid DNA, from stool samples. These biomarkers from dietary components such as plants, mushrooms, and animals, can be amplified and mapped to various reference databases containing eukaryotic sequences. This means that scientists can then identify up to 150 human food species. However, he also noted variable performance for different food groups. This likely can be further improved to identify multiple food groups more accurately. - Microbiome data analysis using machine learning
Machine learning-based methods can transform vast amounts of biological data to capture subtle associations and correlations and help predict biological outcomes. One such application of machine learning came from Efrat Muller from Tel Aviv University, Israel. Her ‘multiview learning’ approach predicts disease-associated microbiome signatures by using different types of data features (aka views) derived from microbiome data, such as the abundance of certain organisms, genes, and metabolites. These views can be processed independently of one another or integrated together to develop disease prediction models. Muller also mentioned that integrating multiple views can help to identify key associative features involved in a given disease.
Similarly, Francesco Asnicar from the University of Trento, Italy, discussed how microbiome data can be used to establish correlations between healthy individuals and those at risk of cardiometabolic conditions. Using machine learning with predictive markers, such as dietary patterns, age, diastolic blood pressure, etc., and relative gut microbial abundance, his research is exploring these correlations.
- From computational conjecture to experimental validations
Computational methods in microbiome analysis provide important biological hypotheses and generate additional data that can be tested experimentally using culture-dependent methods. Harry Sokol from Saint-Antoine Hospital, France, shared how his team used targeted metabolomics and microbiome analysis to identify and compare the abundance of key bacterial metabolites between inflammatory bowel disease and healthy conditions. Likewise, they isolated human gut bacterial strains involved in producing metabolites that suppress inflammatory immune responses and in others involved in tryptophan metabolism (an amino acid that helps produce and maintain the body’s proteins, muscles, enzymes, and neurotransmitters).
Similarly, with leads from microbiome analysis, Ruth Ley from Max Planck Institute for Biology, Germany, showed how both ‘good’ and ‘bad’ intestinal microbes exhibit different activation levels of TLR-5. When TLR5 recognises a protein called flagellin, it triggers a signalling cascade that ultimately activates various immune responses. Ley found that even when both types of microbes carry flagellin protein, “silent flagellins” on ‘good’ gut bacteria allow them to be immune-tolerant.
- The information highway between the gut and brain
Gut microbes are at the centre of bidirectional communication between the nervous system and intestinal functions. This gut-brain axis is so important that dysbiosis can lead to neurodegenerative and psychiatric disorders. Basit Yousuf from the University of Ottawa, Canada, highlighted the role of bacterial extracellular vesicles (extravesicles) in transporting neurotransmitters like serotonin, and bacterial metabolites such as short-chained fatty acids to the nervous system. His work showed that bacterial extravesicles that arise from bacterial cell membranes contain neurotransmitters that cross the epithelial lining and blood-brain barrier in in vitro experiments. This finding has potentially major implications in better understanding health and disease. - The continued quest to clarify medication impacts on the gut
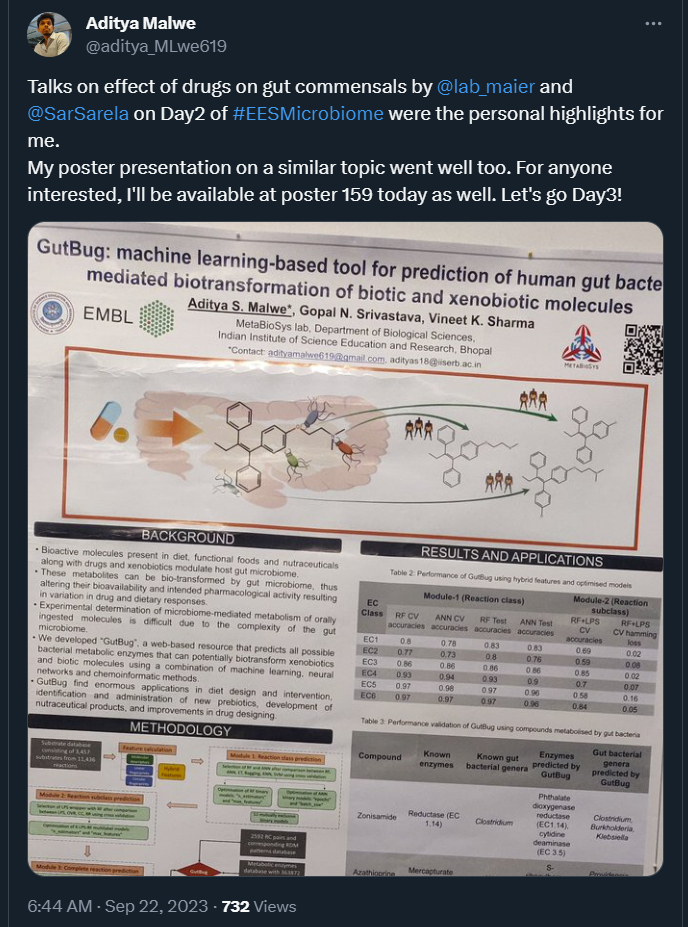
Drugs for various conditions can unintentionally inhibit gut microbes, especially non-antibiotics. Lisa Maier from University of Tubingen, Germany, discussed how this in turn has further negative effects on the human host. While beneficial commensals are susceptible to inhibition by non-antibiotic drugs, many pathogenic γ-proteobacteria, such as Salmonella, seem undeterred. The good news is that as a collective community, gut microbes show some degree of resilience against the inhibitory effects of non-antibiotic drugs.
Sarela Garcia-Santamarina from Instituto de Tecnologia Química e Biológica NOVA, Portugal, provided evidence for the same. Using monocultures and co-cultures of different gut bacteria, she showed that at low drug concentration, resilience is observed but it weakens at higher drug concentrations and relies on a community behaviour using mechanisms such as drug biotransformation and bioaccumulation.
- Study design and sample collection is critical
Many speakers provided detailed sample collection strategies. Sarah Lebeer from the University of Antwerp, Belgium, discussed her ‘Isala project’, involving the vaginal microbiome. She mentioned the importance of making participants feel comfortable and respected during sample collection to obtain intimate and sensitive information that formed crucial metadata for her study. Similarly, Jakob Wirbel from Stanford University, California, USA, raised the idea of participant data ownership. He is working on gut metagenomic profiling of African women and uses feedback sessions to ensure participants feel valued during the entire sample collection process while also overcoming social and logistic challenges.
Confounding factors also can present challenges. Alexandra Zhernakova from University of Groningen, The Netherlands, reported on her collection approach in a long-term project studying early microbiome development in infants. For this study, her team collected samples from mother, father, infant and the environment, generating extensive amounts of metadata which ultimately might or might not prove to impact early microbiome development in infants.
Overall, the symposium provided a good mix of various perspectives on human microbiome research with talks from more established researchers and posters that represented a new generation of researchers aiming to continue the quest for knowledge in the human microbiome field.
Read also about the poster prize winners from ‘The human microbiome’ and find out more about their research in another blog post from the meeting.
The EMBO | EMBL Symposium ‘The human microbiome’ took place between 20-23 September 2023 in Heidelberg, Germany. The meeting brings together experts to discuss the newest findings about the human microbiome and its connection to health and disease.
Did you know that you can become an event reporter and receive a conference fee waiver in exchange? Find out how to do that by visiting our Become an event reporter page.