Read the latest Issue
Supply and demand
EMBL scientists identify proteins that ensure iron balance
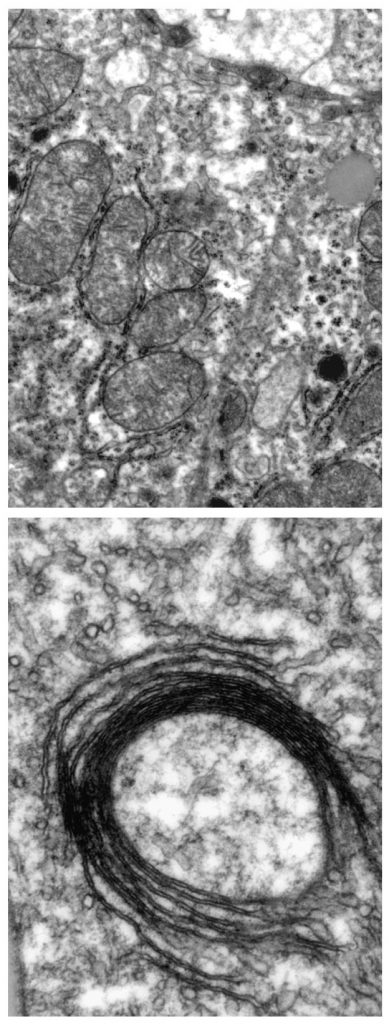
Image credits: Bruno Galy/ EMBL
Most organisms need iron to survive, but too much iron is toxic, and can cause fatal organ failure. The same is true inside cells, where iron balance must also be maintained. In a study published today in Cell Metabolism, scientists at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, have discovered that a group of proteins named IRPs ensure that this iron balance is kept and as such are essential for cell survival. More specifically, they found that IRPs are required for the functioning of mitochondria, the cell’s energy factories.
Mitochondria need iron in order to function, but they also convert iron into other chemical forms used throughout the cell: iron sulphur clusters and haem – one of the building blocks of haemoglobin. Thanks to new mouse models they engineered, the EMBL scientists have been able to selectively shut down IRP function in specific cell types such as hepatocytes, liver cells that carry out multiple vital metabolic functions.
“Mice whose liver cells can’t produce IRPs die of liver failure a few days after birth,” says Bruno Galy, Staff Scientist in Matthias Hentze’s group at EMBL, who spearheaded the work: “The mitochondria in those cells have structural defects and don’t function properly, because they don’t have enough iron.”
Galy and colleagues found that in cells that cannot produce IRPs, the mechanisms for iron export and storage go into over-drive, while iron import is drastically reduced. This combination of factors leads to an iron shortage in the cell. As a consequence, the mitochondria don’t receive enough iron, so they can’t function properly, and can’t make enough haem and iron sulphur clusters available to the cell machinery that depends on them. In short, the role of IRPs is to ensure that there is enough iron available in the cell to sustain mitochondrial iron needs.
“We have indications that this is probably a general process by which most cells control their iron content and secure mitochondrial iron sufficiency” Hentze concludes.
This mechanism for regulating iron balance could be particularly important in cells with very high mitochondrial iron needs, such as red blood cell precursors that manufacture copious amounts of haem for oxygen transport. However, this may well be a double-edged sword. Indeed, there are situations in which mitochondria get iron but are not able to make use of it. The cell interprets this as a sign of mitochondrial iron insufficiency and responds by activating IRPs, which ultimately results in detrimental iron overloading of mitochondria. This may underlie the pathology of several diseases including inherited sideroblastic anaemias – in which cells are unable to incorporate iron into haemoglobin – or the neurodegenerative disorder Friedreich’s ataxia, which the EMBL scientists are currently investigating.