Read the latest Issue
The battle for iron
Scientists in Heidelberg have discovered a new strategy in the cellular version of hunger games, which could spur new approaches to the search for therapies against anaemia of chronic disease.
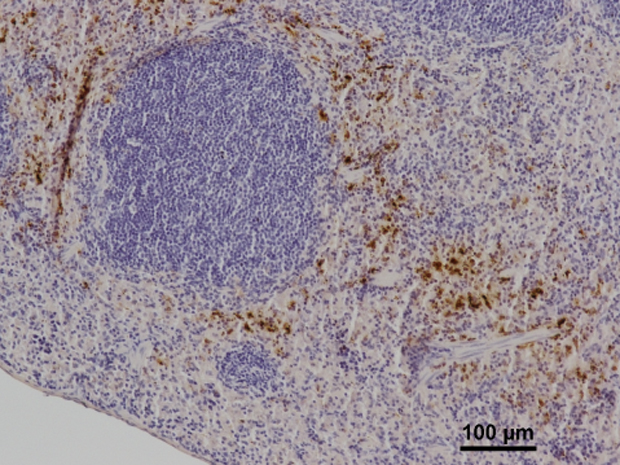
When we think of how we fight disease, the image of cells in our immune system fending off microbial invaders often comes to mind. Another strategy our bodies can employ is to cut off the enemy’s supply lines and effectively starve disease-causing microbes of the iron they need to function. However, this tactic can backfire and cause anaemia if the iron-starved state is sustained for too long, a common problem in chronically ill patients. The search for therapies against this anaemia of chronic disease could now take on new directions, as scientists in the Molecular Medicine Partnership Unit of EMBL and Heidelberg University Clinic have found a hitherto unknown way through which mice starve pathogens of iron.
Mammals keep iron out of reach of invading microbes by storing it in cells like macrophages – white blood cells which, among other things, normally ‘recycle’ the iron from red blood cells back into the bloodstream. When the body is under attack, macrophages respond by decreasing levels of their iron-exporter, ferroportin, thereby sequestering the iron. Scientists knew this decrease in ferroportin could be achieved by increasing levels of hepcidin, a hormone which regulates iron levels. But Claudia Guida, a PhD student in the group jointly led by Matthias Hentze at EMBL Heidelberg and Martina Muckenthaler at Heidelberg University Clinic, found that ferroportin can be dialled down independently of hepcidin, by triggering responses from TLR2 and TLR6, two molecules our immune system uses to detect bacterial components.

“Until now, the main approach to develop treatments for anaemia of chronic disease was to look for anti-hepcidin therapies,” says Hentze. “Our findings provide an alternative approach, which is especially relevant because not all patients with anaemia of chronic disease have increased hepcidin levels.”
Why do these cells have two ways of decreasing ferroportin levels? “It could be that this is such an important response, that organisms have evolved a fail-safe, so that if one response fails, they have the other; or it could be a way of broadening the spectrum of what you’re protected against: the hepcidin response might be triggered by some pathogens, and the TLR2/TLR6 response could be activated by others,” says Muckenthaler, “or it could be that this TLR2/TLR6 response we found is a first line of defense, and then the hepcidin response ‘kicks in’ later.”
Hentze, Muckenthaler and colleagues are now investigating exactly what causes ferroportin levels to decrease when TLR2 and TLR6 are activated. As well as informing the search for therapies, this could one day help to develop tests to determine if a patient’s anaemia is caused by problems in his or her TLR2/TLR6 response.
How it all came about
Matthias Hentze discusses how the team discovered this novel iron-sequestering strategy thanks to a screen developed with EMBL alumnus Michael Boutros, now at the German Cancer Research Centre.