
Read the latest Issue
EMBL researchers and collaborators secure funding for four projects
Curiosity-driven fundamental research is a seedbed for innovative ideas and technologies that can benefit society. But without effective mechanisms for taking these technologies and scaling them up for wider application in the scientific and commercial spheres, many great ideas might never make it out of the lab.
The ATTRACT consortium was established to address this challenge and create a new European model of open innovation. The initiative – funded by the European Commission’s Horizon 2020 research and innovation programme – brings Europe’s fundamental research and industry communities into a shared space that fosters creativity and collaboration. Together, they will focus on developing cutting-edge detection and imaging technologies, and will apply these technologies to address key societal challenges in areas such as medical diagnostics, climate change and environmental monitoring.
In the first phase of ATTRACT funding, 170 projects – selected from over 1200 proposals – were awarded seed grants of €100,000 each. EMBL is a collaborator in four of the funded projects, and two of these are being led by EMBL researchers. They have one year to prove the scientific merit and innovation potential of their technologies, before presenting their results at a conference in Brussels in autumn 2020. An independent committee will then decide which of the projects will receive additional funding under ATTRACT Phase 2. The clock is ticking.
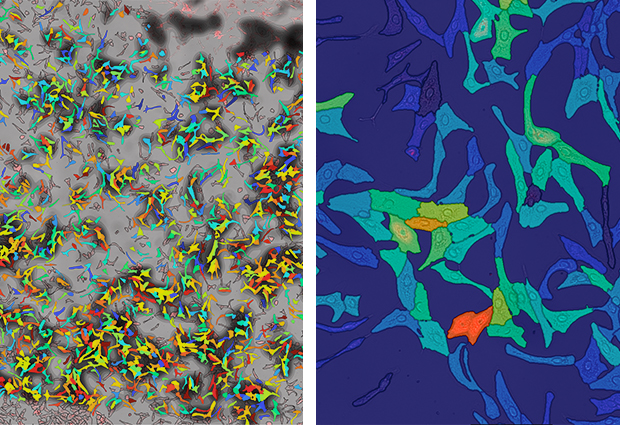
EMBL team leader Theodore Alexandrov develops tools to investigate spatial aspects of metabolism. His team has developed a technology to detect small molecules, metabolites, and lipids in individual cells, and link this molecular information to cell states and spatial behaviour. Alexandrov explains: “We developed a method to overlay microscopy images of cells with their molecular profiles sampled using a laser. Just a few years ago, this would have been in the realm of science fiction, because of how tiny cells are. New discoveries about metabolic states of individual cells can be beneficial in applications where a single cell can be a game changer, such as in cancer, immunity or infection.” The method has received a lot of interest from funding agencies and potential collaborators, both in research and industry. This got Alexandrov and his team thinking about commercialisation. ATTRACT gave them an opportunity to pursue this.
“We need pragmatic, applied developments to make this method work outside our lab, and to make it more high-throughput,” says Alexandrov, “and this is normally hard to get funding for.” Together with DKFZ (the German Cancer Research Center), a key collaborator in the project, the team is investigating how a high-fat diet triggers inflammation in the liver, and how drugs can help curb this inflammation and thus prevent the formation of liver cancer. This technology has the potential to advance biomedical discovery in cancer, immunity and infection, as well as to facilitate the development of new drugs.
Capturing highly dynamic biological processes on large spatial scales – such as a beating heart, or the activity of neurons in the brain – remains one of the key challenges for microscopy. Light-sheet microscopy – a technique that allows high-quality three-dimensional imaging – is fast, but for these types of applications it needs to be much faster. Lars Hufnagel and his group at EMBL are joining forces with collaborators from EPFL – a technical university in Lausanne, Switzerland – and EMBL spin-off Luxendo to make this happen.
“My group at EMBL are building the light-sheet microscope and all the automation,” explains Hufnagel. “The group at EPFL are at the forefront of high-speed detector development – they build sensor arrays that are 1000 to 100,000 times faster than the cameras we’re currently using. They’ll supply the detectors and help us adapt and integrate them to work for our setup. Luxendo will get involved later, once we have the first prototype and are ready for commercialisation. They can help us get the technology into the market, and to the users.” The proposed technology will exceed the capabilities of existing volumetric imaging techniques in terms of detection speed, spatial resolution and image quality.
Fluorescence microscopy is the most commonly used analytical technique in the field of optogenetics – using light to control the behaviour of cells in living tissue. But as Kathrin Brenker of opto biolabs – a spin-off from the University of Freiburg, Germany – explains, “I did this during my PhD and I found it’s a very slow way of analysing cells. So I started developing illumination devices for flow cytometry and optogenetics.” Flow cytometry is a technique that uses light scattering to analyse the physical and chemical properties of cells or particles as they flow – suspended in a fluid – across a beam of laser light. Brenker and colleagues at opto biolabs and the University of Freiburg are now collaborating with Malte Paulsen, head of EMBL’s Flow Cytometry Core Facility, and his team to develop an illumination device that allows them to combine optogenetics with fluorescence-activated cell sorting – an application of flow cytometry in which cells are sorted according to their light scattering and fluorescence characteristics.
“Malte is a genius when it comes to flow cytometry, whereas our expertise is more in the area of LEDs, light and optics. Bringing these two things together will help us build a working prototype,” says Brenker. “We’re hoping to have one by the end of the year.” This technology could help translate fundamental research into therapeutic applications such as individualised cancer immunotherapy.
Cryo-electron tomography (cryo-ET) can produce images of large molecular complexes at almost the level of individual atoms. However, imaging whole organisms or tissues and then identifying key features and physically extracting them for high-resolution imaging presents a technical challenge: how to deal with the shift in scale from millimetres to nanometres. This is the challenge that Marco Endrizzi and his team at University College London are tackling in collaboration with Yannick Schwab’s team in the Cell Biology and Biophysics Unit at EMBL.
Biological samples are typically prepared for electron microscopy by staining them with solutions containing atoms of heavy metals, which are opaque to electrons. The proposed method avoids this process and instead uses pure, thin samples that have been rapidly frozen to prevent the formation of ice crystals. Without the staining it’s no longer possible to rely on variations in the absorption of X-rays as they pass through different parts of a sample, to image its internal structure. Instead, differences in the phase of the X-rays – a property that describes where a wave is in its cycle of peaks and troughs – are the basis for Endrizzi’s imaging technique. The team has already conducted a feasibility study at a synchrotron, which is a large-scale particle accelerator that produces powerful X-rays. “The aim now is to translate this into a tool that could sit next to the electron microscope in the lab,” says Endrizzi.
Looking for past print editions of EMBLetc.? Browse our archive, going back 20 years.
EMBLetc. archive