Read the latest Issue
How cells keep in shape
Scientists elucidate a molecular mechanism that plays a key role in giving a cell its shape
Cells in our body come in various shapes and sizes. Each cell is shaped in such a way as to optimise it for a specific function. When things go wrong and a cell does not adopt its dedicated shape, its function can be impaired and the cell can cause problems in the body. Researchers at the European Molecular Biology Laboratory (EMBL) and the Institute for Atomic and Molecular Physics (AMOLF), The Netherlands, have now decoded a molecular mechanism that plays an important role in the development of a cell’s shape. In this week’s issue of Nature they report a new experimental approach that sheds light on the interaction between proteins and the cell’s skeleton.
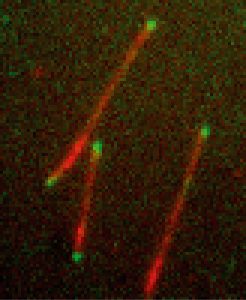
That each cell type has its unique shape is due to its cytoskeleton, an internal scaffold built of protein filaments. Especially important are microtubules, dynamic filaments that constantly grow and shrink. Their spatial organisation inside cells depends on a variety of regulator proteins, some of which only interact with the growing ends of these filament. How these so called plus-end tracking proteins recognise the dynamic structure of a growing microtubule end is a long-standing puzzle. Researchers in the groups of Thomas Surrey and Damian Brunner at EMBL and of Marileen Dogetrom at AMOLF have now developed the first method that allows to simultaneously study multiple plus-end tracking molecules, so called +TIPs, in the test tube.
“+TIPs specifically bind to the fast-growing plus end of a microtubule and follow it as it grows. They act as a plus-end label so that other proteins know where to bind to regulate the filament’s stability,” says Surrey. “For years it has been impossible to reconstitute this behaviour in a test tube. Our new system now revealed which proteins need to be present for plusend tracking and what the underlying mechanisms are.”
Applying the new method they succeeded in dissecting a minimal molecular system consisting of three end tracking proteins from yeast cells. The proteins were labelled with fluorescence to monitor their behaviour with a microscope. This procedure revealed that one of the proteins has the ability to recognise the specific structure of the growing microtubule tip, binds to it and acts as a loading platform for the other two proteins. The inherent motor activity of one of the other two proteins, which allows it to walk along microtubules, contributes to the ability of the molecular system to follow growing microtubule ends selectively.
“The great advantage of our new assay is that it can be applied to all kinds of other proteins that interact with microtubules,” says Peter Bieling, who carried out the research in Surrey’s lab. “It is a powerful approach that will advance our understanding of the large variety of different microtubule end tracking proteins and can shed light on their mechanics and functions.”