This process of translation is essential for life and is present in all organisms. While their basic working mechanisms are similar, ribosomes in bacteria (prokaryotes) and higher organisms (eukaryotes) have different structures. These differences present an important opportunity: around half of the antibiotics currently in use selectively target the prokaryotic ribosome to disrupt protein synthesis and stop microbial attack. Understanding the structure and mechanism of the translational machinery can provide key information for research on new antibiotics and on antibiotic resistance – an issue of critical importance that is threatening effective prevention and treatment of infectious diseases.
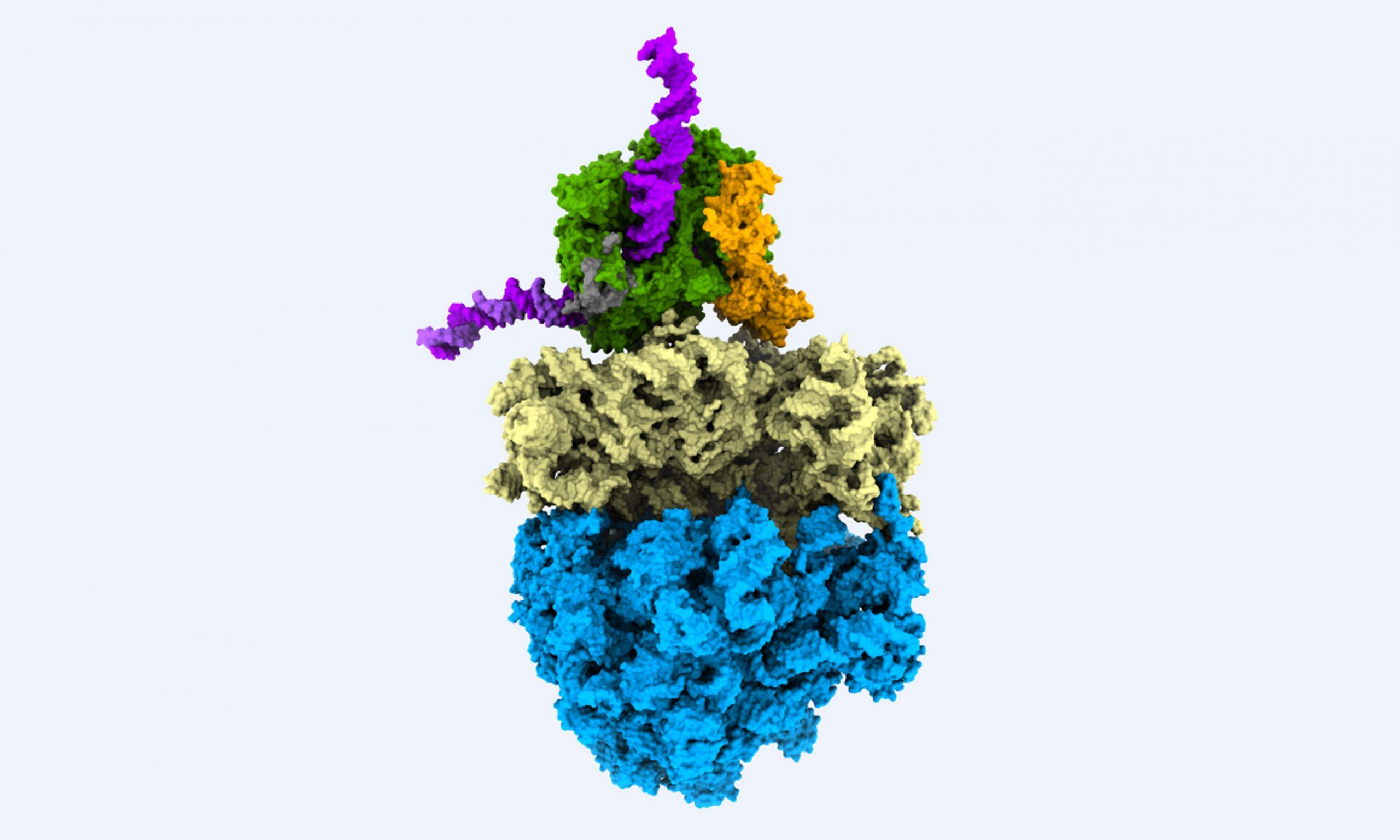
Ribosomes were first described as cellular organelles in the 1950s. Over the next decade, scientists managed to work out the central role that ribosomes play in all living cells, but they were unable to determine their structure or precise mechanism. In the 1970s, studies using classical electron microscopy provided the first views of the ribosomal architecture, albeit at fairly low resolution. To improve on the 'blobby' electron microscope image, the researchers would need to use X-ray crystallography. This technique requires the large molecular assembly of the ribosome to be arranged into a regular order -- a crystal -- a feat that seemed well beyond the achievable at the time.
In the late 1970s, Israeli crystallographer Ada Yonath decided to tackle ribosomal crystallography, more than a decade before others entered the field. Throughout the 1980s and early 1990s, Yonath worked to coax ribosomes into more ordered lattices and tested the resulting crystals using powerful X-rays. This is where scientists at EMBL Hamburg and EMBL Grenoble were able to help Yonath to tackle this seemingly impossible task.
Scientific breakthroughs are often made possible only by technological innovation. Using beamlines at synchrotrons, such as the EMBL beamline at the German Electron Synchrotron Radiation Facility (DESY) in Hamburg, Yonath not only showed success in growing ribosomal crystals, but also developed a method for freezing biological crystals that allowed them to survive the intense beams for longer. The technique, termed cryo-crystallography, enabled Yonath to obtain crystallographic data on ribosomes -- and has since become routine in structural studies of macromolecules.
The breakthrough occurred in 2000 and 2001, when the hard work of Yonath and other groups paid off: together, they revealed the structure of ribosomal particles at atomic resolution. In recognition of this achievement, Yonath was awarded the 2009 Nobel Prize in Chemistry alongside two other crystallographers. Yonath worked on the EMBL beamlines during her time as head of a unit of the Max Planck Institute for the Structure and Dynamics of Matter at DESY from 1986 to 2004. She highlighted the crucial contribution of EMBL in providing experimental equipment for her studies:
