- STELLARIS 8 STED Falcon
- THUNDER Imager Live Cell
- MultiView SPIM
- STELLARIS 8 DIVE Falcon
- DMi8 S with scanner and TIRF module
STELLARIS 8 STED Falcon
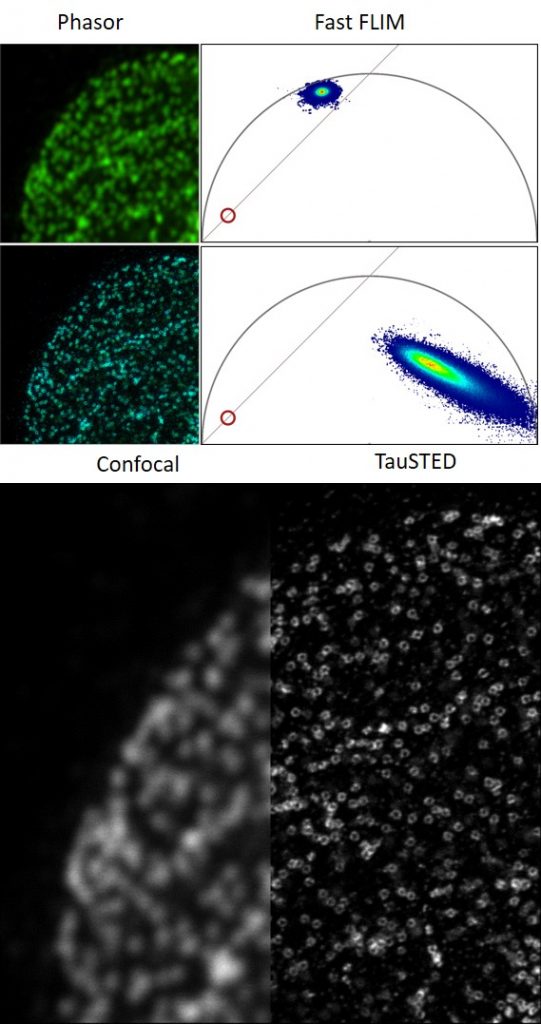
STELLARIS 8 STED with TauSTED from Leica Microsystems allows the study of multiple dynamic events simultaneously and investigation of molecular relationships and mechanisms within the cellular context.
FALCON (FAst Lifetime CONtrast) includes phasors for quantitative FLIM analysis. It is a fully integrated solution for fluorescence lifetime imaging (FLIM) and enables video-rate image acquisition for rapid kinetic studies in live cells.
Features
- Resolving molecular relationships in specimens
- Multiple simultaneous events can be studied at the nanoscale
- 2D and 3D STED imaging
- Significantly reduced light doses due to TauSTED
- Advanced STED-FLIM options with STED and FALCON (full FLIM quantitative tools, phasor FLIM, species separation based on phasor analysis)
- Advanced STED-FCS options
- Fast validation of results on a single platform, from confocal to super-resolution LIGHTNING and STED
- With FALCON it is possible to:
- follow fast molecular interactions via FLIM-FRET (Förster resonance energy transfer)
- use biosensors to detect microenvironmental changes, such as pH or ion concentration
- apply lifetime contrast to separate multiple fluorophores
- Analysis using phasor FLIM provides a 2D visualisation of lifetime components. With FLIM phasors you can follow microenvironmental changes, select components to multiplex signal (phasor separation), and determine FRET efficiency
Specifications
- Five spectrally tunable Power HyD sensitive photon counting detectors (2 HyD S, 2 HyD X, 1 HyD R)
- Confocal: tunable White Light Lasers (WLL) 440-790 nm, 405 nm STED depletion: 592 nm, 660 nm, 775 nm
- 8kHz Resonant Scanner
- Total system dead-time: 1.5 ns
- TauSTED: Tunable resolution based on lifetime (depending on sample and fluorophore: <30 nm (lateral) and <100 nm (axial). Automatic lifetime-based background suppression algorithm. Light dose reduction (WLL excitation) for all STED lines (592, 660, 775 nm). Available for 2D and 3D STED in live and in fixed specimens, also for multicolor applications. Automated workflow integrated in the LAS X software
- The STED WHITE glycerol and water objective lenses with motCORR technology provide adaptive optical correction for aberrations introduced by sample inhomogeneities and refractive-index mismatch. The STED WHITE objective lenses provide a working distance of 300 µm:
- HC PL APO 86x/1.20 W motCORR STED WHITE
- HC PL APO 93x/1.30 GLYC motCORR STED WHITE
- HC PL APO 100x/1.40 OIL STED WHITE

THUNDER Imager Live Cell
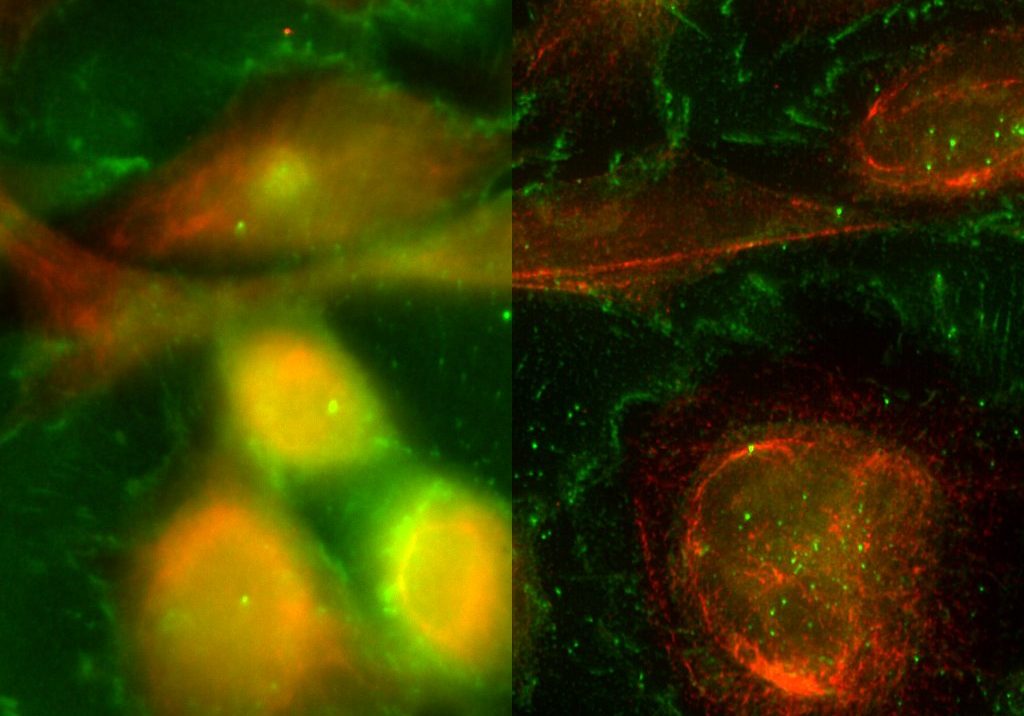
THUNDER is an opto-digital technology from Leica Microsystems that uses the Computational Clearing method for haze removal to generate high-resolution and high-contrast images.
A THUNDER Imager Live Cell provides a complete microscopy imaging system for minimally invasive and precise live-cell imaging. High-speed and/or long term examination of specimens, e.g. cell cultures and organoids, can be performed at low phototoxicity and photobleaching.
Features
- sCMOS technology
- Efficient removal of out-of-focus blur in real time with Computational Clearing
- Automation of the imaging and analysis workflow within the LAS X software
- Further processing and reconstruction using the Aivia software platform
Specifications
- Based on the fully motorised, high-end, inverted research microscope DMi8
- High-speed positioning with the Quantum stage and Synapse real-time controller
- High-speed illumination with a multi-line LED light source
- Fast switching external filter wheel
- Adaptive Focus Control (AFC) with closed loop focus
- Climate chamber ensures optimal physiological conditions for living cells

MultiView SPIM
The MultiView-SPIM (Selective plane illumination microscope) is an advanced light sheet fluorescence microscope utilising multiple orthogonal optical paths for in vivo imaging of small embryos, organisms and cell cultures. The MultiView-SPIM available at the EMBL IC has originally been developed by the lab of Lars Hufnagel at EMBL and further developed by the lab of Robert Prevedel at EMBL. Owing to its plane wise illumination confinement and fast imaging on multiple cameras, the MultiView-SPIM is able to capture developmental dynamics with minimal photodamage to the living subjects.+
Features
- Multi-view reconstruction via fusion of multiple camera views
- Environmental control (Temperatures, CO2 etc.)
- Variable magnification 22 – 33x (600 – 400 um field of view)
- Sub-micron resolution for superficial imaging, performance at depth dependent on sample optical properties.
- Imaging typically at 50 – 100 frames per second, volume rate typically 0.1 – 1 volume per second
- Multi-color imaging achieved sequentially with fast filter wheels
- Live samples can be imaged over several days
Specifications
- Light sheets available at 488, 515, 594 nm (and 685, 808 nm on request)
- Light sheets generated by beam scanning and combined with camera-based confocal line detection to reject blur and enhance contrast
- Motorised x,y,z and rotation stages
- Sample mounting in refractive index matching tubes (FEP n = 1.33) or extruded in gelated column from glass capillaries
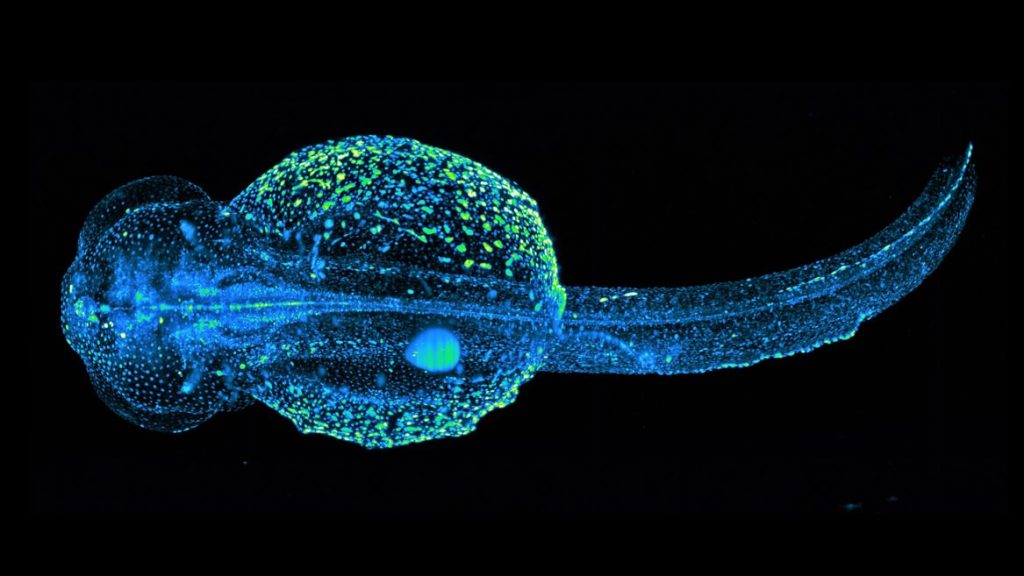
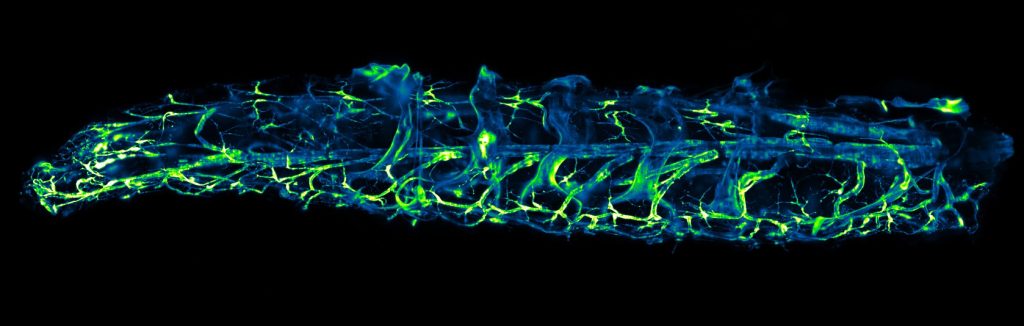
STELLARIS 8 DIVE Falcon
STELLARIS 8 DIVE with the 4Tune detection system is a spectrally tunable multiphoton microscope from Leica Microsystems. Four non-descanned spectrally flexible channels provide multicolor multiphoton imaging at > 1 mm depth.
Lifetime-based information can be directly obtained in addition to distinguish spectrally similar fluorophores, separate second harmonic (SHG) signals from fluorescence or visualise the metabolic state of cells. Lifetime analysis and quantification can be done FALCON.
Features
- 4 spectrally tunable NDD detectors in the visible range (380 – 800 nm)
- Qualitative and semi-quantitative lifetime-based information with TauSense
- Fully quantitative FLIM analysis with FALCON, including phasors
Specifications
- Laser lines: IR: tunable 680 – 1080 nm and 680 – 1300 nm, fixed 1040 nm; Confocal: White Light Lasers (WLL) 440 – 790 nm, 405 nm, and 488 nm
- Four NDD channels equipped with Power HyD X (tunable from 380 – 800 nm), five spectrally tunable internal counting detectors (3 HyD S, 1 HyD X, and 1 HyD R)
- Upright confocal fixed-stage (DM6 CFS) stand furnished with Scientifica scanning stage

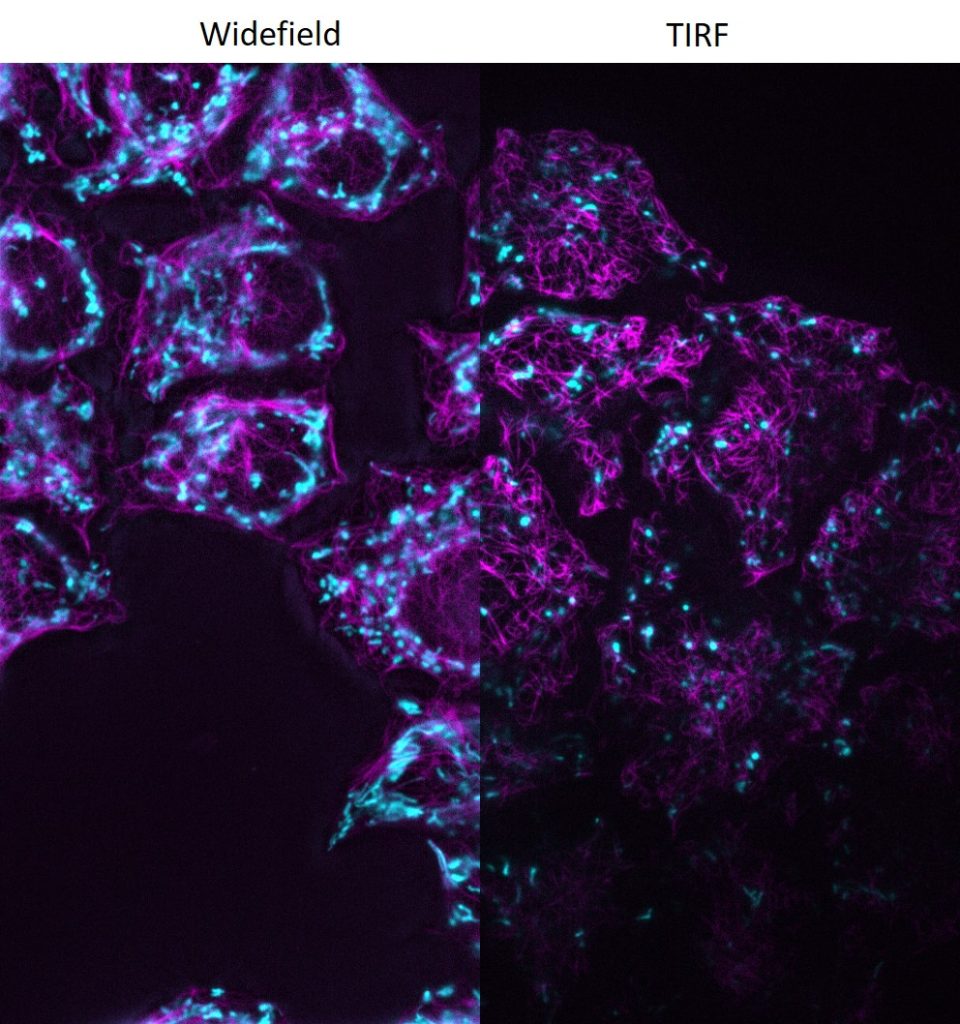
DMi8 S with scanner and TIRF module
DMi8 S from Leica Microsystems is a live cell imaging system to execute advanced experiments with imaging modalities like photomanipulation or TIRF, both simultaneously or sequential. For dynamic processes at the cell surface TIRF (Total Internal Reflection Fluorescence) microscopy is the method of choice to visualize single molecules with super-resolution by maximizing the fluorescent signal- to-noise-ratio. Furthermore, the system can be set-up for multispectral photomanipulation techniques like FRAP, acceptor bleaching, activation, optogenetics, DNA damage, or ablation within one single experiment. Imaging specialists are on site to support your particular application.
Features
- Infinity TIRF module with azimuth shifting capability to optimise the illumination field.
- Infinity scanner for advanced multispectral photomanipulation applications (enables to bleach, cut, activate, stimulate).
- LAS X Navigator software to create quick overviews of the sample and identify the important details instantly. High resolution image acquisition can be set up using templates for slides, dishes, and multi-well plates.
Specifications
- Fully motorised DMi8 inverted research microscope with integrated real-time controller to operate the system with microsecond precision
- Adaptive Focus Control (AFC)
- Equipped with a high-end sCMOS camera